|
Paroxypropione
Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed. It was first synthesized in 1902. The antigonadotropic properties of the drug were discovered in 1951 and it entered clinical use shortly thereafter. Pharmacology Pharmacodynamics Paroxypropione is closely related structurally to ''p''-hydroxybenzoic acid and parabens such as methylparaben, and also bears a close resemblance to diethylstilbestrol (which, in fact, produces paroxypropione as an active metabolite) and alkylphenols like nonylphenol, all of which are also estrogens. The drug possesses relatively low affinity for the estrogen receptor and must be given at high dosages to achieve significant estrogenic and antigonadotropic effects, for instance, 0.8 to 1.6 g/day. It possesses 0.1% of the estrogenic activity and less than 0.5% of the antigonadotropic potency of estrone. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylstilbestrol
Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection. DES is an estrogen, or an agonist of the estrogen receptors, the biological target of es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Estrogen
A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity. Pharmacology Nonsteroidal estrogens act as agonists of the estrogen receptors, ERα an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
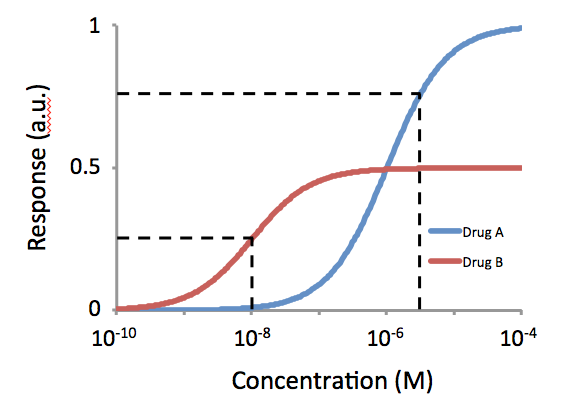
Potency (pharmacology)
In the field of pharmacology Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemi ..., potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency ( meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ketones
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antioxidants
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricants, to prevent oxidation, and to foods to prevent spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress. The only dietary antioxidants are vitamins A, C, and E, but the term ''antioxidant'' has also been applied to numerous other dietary compounds that only have antioxidant properties in vitro, with little evidence for antioxidant properties in vivo. Dietary supplements marketed as antioxidants have not been shown to maintain health or prevent disease in humans. History As part of their adaptation from marine life, terrestrial plants began producing non-marine anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abandoned Drugs
Abandon, abandoned, or abandonment may refer to: Common uses * Abandonment (emotional), a subjective emotional state in which people feel undesired, left behind, insecure, or discarded * Abandonment (legal), a legal term regarding property ** Child abandonment, the extralegal abandonment of children ** Lost, mislaid, and abandoned property, legal status of property after abandonment and rediscovery * Abandonment (mysticism) Art, entertainment, and media Film * ''Abandon'' (film), a 2002 film starring Katie Holmes * ''Abandoned'' (1949 film), starring Dennis O'Keefe * ''Abandoned'' (1955 film), the English language title of the Italian war film ''Gli Sbandati'' * ''Abandoned'' (2001 film), a Hungarian film * ''Abandoned'' (2010 film), starring Brittany Murphy * ''Abandoned'' (2015 film), a television movie about the shipwreck of the ''Rose-Noëlle'' in 1989 * ''Abandoned'' (2022 film), starring Emma Roberts * ''The Abandoned'' (1945 film), a 1945 Mexican film * ''The Ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dienestrol
Dienestrol (, ) (brand names Ortho Dienestrol, Dienoestrol, Dienoestrol Ortho, Sexadien, Denestrolin, Dienol, Dinovex, Follormon, Oestrodiene, Synestrol, numerous others), also known as dienoestrol (), is a synthetic nonsteroidal estrogen of the stilbestrol group which is or was used to treat menopausal symptoms in the United States and Europe. It has been studied for use by rectal administration in the treatment of prostate cancer in men as well. The medication was introduced in the U.S. in 1947 by Schering as Synestrol and in France in 1948 as Cycladiene. Dienestrol is a close analogue of diethylstilbestrol. It has approximately 223% and 404% of the affinity of estradiol at the ERα and ERβ, respectively. Dienestrol diacetate Dienestrol diacetate (brand names Faragynol, Gynocyrol, others) is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of dienestrol. See also * List of estrogen esters § Esters of ot .. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as ''reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing ("work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precursor (chemistry)
In chemistry, a precursor is a compound that participates in a chemical reaction that produces another compound. In biochemistry, the term "precursor" often refers more specifically to a chemical compound preceding another in a metabolic pathway, such as a protein precursor. Illicit drug precursors In 1988, the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances introduced detailed provisions and requirements relating the control of precursors used to produce drugs of abuse. In Europe the Regulation (EC) No. 273/2004 of the European Parliament and of the Council on drug precursors was adopted on 11 February 2004. ( European law on drug precursors) Illicit explosives precursors On January 15, 2013, the Regulation (EU) No. 98/2013 of the European Parliament and of the Council on the marketing and use of explosives precursors was adopted. The Regulation harmonises rules across Europe on the making available, introduction, possession a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fries Rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids. It involves migration of an acyl group of phenol ester to the aryl ring. The reaction is ortho and para selective and one of the two products can be favoured by changing reaction conditions, such as temperature and solvent. Mechanism Despite many efforts, a definitive reaction mechanism for the Fries rearrangement has not been determined. Evidence for inter- and intramolecular mechanisms have been obtained by crossover experiments with mixed reactants. The reaction progress is not dependent on solvent or substrate. A widely accepted mechanism involves a carbocation intermediate. In the first reaction step a Lewis acid for instance aluminium chloride co-ordinates to the carbonyl oxygen atom of the acyl group. This oxygen atom is more electron rich than the phenolic oxygen atom and is the prefe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropionate
Phenylpropanoic acid or hydrocinnamic acid is a carboxylic acid with the formula C9H10O2 belonging to the class of phenylpropanoids. It is a white, crystalline solid with a sweet, floral scent at room temperature. Phenylpropanoic acid has a wide variety of uses including cosmetics, food additives, and pharmaceuticals. Preparation and reactions Phenylpropanoic acid can be prepared from cinnamic acid by hydrogenation. Originally it was prepared by reduction with sodium amalgam in water and by electrolysis. A characteristic reaction of phenylpropanoic acid is its cyclization to 1-indanone. When the side chain is homologated by the Arndt–Eistert reaction, subsequent cyclization affords 2-tetralone, derivatives. Uses Phenylpropanoic acid is widely used for flavoring, food additives, spices, fragrance, and medicines as it acts as a fixative agent, or a preservative. Food industry Phenylpropanoic acid is used in the food industry to preserve and maintain the original aroma qual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)