|
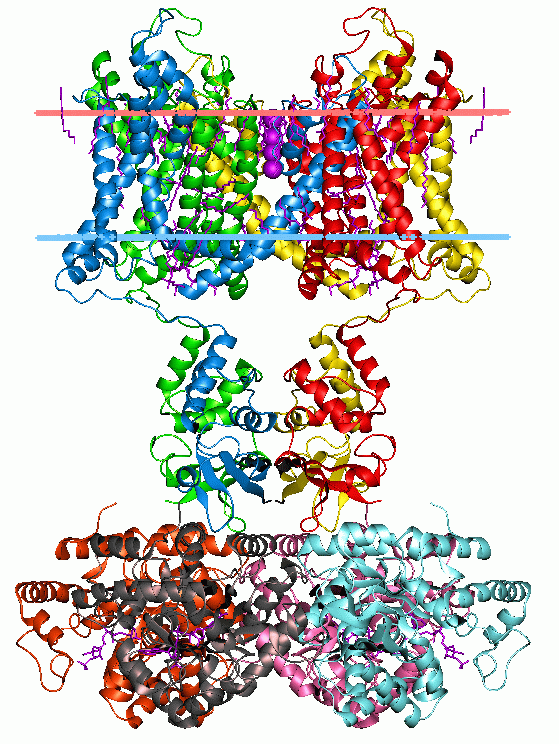
PTS-GFL Superfamily
The phosphotransferases system (PTS-GFL) superfamily is a Protein superfamily, superfamily of Phosphotransferase, phosphotransferase enzymes that facilitate the transport of glucose, glucitol (G), fructose (F) and lactose (L). Classification has been established through phylogenic analysis and bioinformatics. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC). The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superfamilies, that include the (1) Glucose (Glc),(2) Mannose (Man), (3) Ascorbate-Galactitol (Asc-Gat) and (4) Dihydroxyacetone (Dha) superfamilies. The four families that make up the PTS-GFL superfamily include: 4.A.1– The PTS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Superfamily
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred (see homology (biology), homology). Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent (due to low sequence similarity). Superfamilies typically contain several protein families which show sequence similarity within each family. The term ''protein clan'' is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems. Identification Superfamilies of proteins are identified using a number of methods. Closely related members can be identified by different methods to those needed to group the most evolutionarily divergent members. Sequence similarity Historically, the similarity of different amino acid sequences has been the most common method of inferring Sequence homology, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digital Object Identifier
A digital object identifier (DOI) is a persistent identifier or handle used to uniquely identify various objects, standardized by the International Organization for Standardization (ISO). DOIs are an implementation of the Handle System; they also fit within the URI system ( Uniform Resource Identifier). They are widely used to identify academic, professional, and government information, such as journal articles, research reports, data sets, and official publications. DOIs have also been used to identify other types of information resources, such as commercial videos. A DOI aims to resolve to its target, the information object to which the DOI refers. This is achieved by binding the DOI to metadata about the object, such as a URL where the object is located. Thus, by being actionable and interoperable, a DOI differs from ISBNs or ISRCs which are identifiers only. The DOI system uses the indecs Content Model for representing metadata. The DOI for a document remains fixed over t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transport Proteins
A transport protein (variously referred to as a transmembrane pump, transporter, escort protein, acid transport protein, cation transport protein, or anion transport protein) is a protein that serves the function of moving other materials within an organism. Transport proteins are vital to the growth and life of all living things. There are several different kinds of transport proteins. Carrier proteins are proteins involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Carrier proteins are integral membrane proteins; that is, they exist within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion (i.e., passive transport) or active transport. These mechanisms of movement are known as carrier-mediated transport. Each carrier protein is designed to recognize only one substance or one group of very similar substances. Research ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Transporters
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Proteins
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PubMed Identifier
PubMed is a free search engine accessing primarily the MEDLINE database of references and abstracts on life sciences and biomedical topics. The United States National Library of Medicine (NLM) at the National Institutes of Health maintain the database as part of the Entrez system of information retrieval. From 1971 to 1997, online access to the MEDLINE database had been primarily through institutional facilities, such as university libraries. PubMed, first released in January 1996, ushered in the era of private, free, home- and office-based MEDLINE searching. The PubMed system was offered free to the public starting in June 1997. Content In addition to MEDLINE, PubMed provides access to: * older references from the print version of ''Index Medicus'', back to 1951 and earlier * references to some journals before they were indexed in Index Medicus and MEDLINE, for instance ''Science'', ''BMJ'', and ''Annals of Surgery'' * very recent entries to records for an article before it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Standard Serial Number
An International Standard Serial Number (ISSN) is an eight-digit serial number used to uniquely identify a serial publication, such as a magazine. The ISSN is especially helpful in distinguishing between serials with the same title. ISSNs are used in ordering, cataloging, interlibrary loans, and other practices in connection with serial literature. The ISSN system was first drafted as an International Organization for Standardization (ISO) international standard in 1971 and published as ISO 3297 in 1975. ISO subcommittee TC 46/SC 9 is responsible for maintaining the standard. When a serial with the same content is published in more than one media type, a different ISSN is assigned to each media type. For example, many serials are published both in print and electronic media. The ISSN system refers to these types as print ISSN (p-ISSN) and electronic ISSN (e-ISSN). Consequently, as defined in ISO 3297:2007, every serial in the ISSN system is also assigned a linking ISSN ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphotransferase System
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm. The PTS system uses active transport. After the translocation across the membrane, the metabolites transported are modified. The system was discovered by Saul Roseman in 1964. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC).The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superfamilies, that include the (1) Glucose (Glc),(2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphotransferase
Phosphotransferases are a category of enzymes ( EC number 2.7) that catalyze phosphorylation reactions. The general form of the reactions they catalyze is: :A-P + B \rightleftharpoons B-P + A Where ''P'' is a phosphate group and A and B are the donating and accepting molecules, respectively. Classification Phosphotransferases are generally classified according to the acceptor molecule. , Classification in this article follows the rules of Enzyme Nomenclature of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). *EC 2.7.1 Phosphotransferases with an as acceptor *EC 2.7.2 Phosphotransferases with a |
PTS Glucitol Family
The PTS Glucitol (Gut) FamilyTC# 4.A.4consists only of glucitol-specific porters, but these occur both in Gram-negative and Gram-positive bacteria. It is part of the PTS-GFL superfamily. Structure IIGut of ''Escherichia coli ''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...'' consists of three proteins, a IIA protein, a putative 4 TMS IIC2 protein and a putative 4 TMS IIC1 protein. The N- and C-termini as well as the IIB domain may thereby be localized to the cell cytoplasm, but the topology has not been established experimentally. IIAGut is believed to be phosphorylated on a histidyl residue, while IIBGut is probably phosphorylated on a cysteyl residue. However, these possibilities have not been demonstrated experimentally. References {{CCBYSASource, sourcepath=http://tcdb.org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTS Lactose-N,N'-Diacetylchitobiose Family
The PTS Lactose-N,N’-Diacetylchitobiose (Lac) FamilyTC# 4.A.3 includes several sequenced lactose porters of Gram-positive bacteria, as well as the ''Escherichia coli'' and '' Borrelia burgdorferi'' N,N'-diacetylchitobiose (Chb) porters. It is part of the PTS-GFL superfamily. The former can transport aromatic β-glucosides and cellobiose, as well as Chb. However, only Chb induces expression of the ''chb'' operon. Structure While the Lac porters consist of two polypeptide chains (IIA and IICB), the Chb porters of ''E. coli'' and ''B. burgdorferi'' consist of three (IIA, IIB and IIC). In ''E. coli,'' the IIAChb protein has been shown to form a stable dimer both when phosphorylated and when unphosphorylated. The IIC domains of these permeases are believed to have a uniform topology with 8 TMSs. Lac porters in ''E. coli'' In ''E. coli'', the IIBChb is a monomer. Two IIBChb monomers associate with the IIAChb dimer. The structure of the IIB domain of the Chb porter has been determine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |