|
PPADS
PPADS (pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid) is a selective purinergic P2X antagonist An antagonist is a character in a story who is presented as the chief foe of the protagonist. Etymology The English word antagonist comes from the Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, riv .... It is able to block contractions of rabbit vas deferens induced by ATP or α,β,methylene-ATP. It appears to be relatively selective for P2X receptors, having no appreciable activity at α1 adrenergic, muscarinic M2 and M3, histamine H1, and adenosine A1 receptors. References Azo compounds Organophosphates Pyridines Sulfonic acids {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P2X Receptor
The ATP-gated P2X receptor cation channel familyTC# 1.A.7, or simply P2X receptor family, consists of cation-permeable ligand-gated ion channels that open in response to the binding of extracellular adenosine 5'-triphosphate ( ATP). They belong to a larger family of receptors known as the ENaC/P2X superfamily. ENaC and P2X receptors have similar 3-D structures and are homologous. P2X receptors are present in a diverse array of organisms including humans, mouse, rat, rabbit, chicken, zebrafish, bullfrog, fluke, and amoeba. Physiological roles P2X receptors are involved in a variety of physiological processes, including: * Modulation of cardiac rhythm and contractility * Modulation of vascular tone * Mediation of nociception, especially chronic pain * Contraction of the vas deferens during ejaculation * Contraction of the urinary bladder during micturition * Platelet aggregation * Macrophage activation * Apoptosis * Neuronal-glial integration Tissue distribution P2X receptors ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antagonist
An antagonist is a character in a story who is presented as the chief foe of the protagonist. Etymology The English word antagonist comes from the Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, rival," which is derived from ''anti-'' ("against") and ''agonizesthai'' ("to contend for a prize"). Types Heroes and villains The antagonist is commonly positioned against the protagonist and their world order. While most narratives will often portray the protagonist as a hero and the antagonist as a villain, like Harry Potter and Lord Voldemort in '' Harry Potter'', the antagonist does not always appear as the villain. In some narratives, like Light Yagami and L in '' Death Note'', the protagonist is a villain and the antagonist is an opposing hero. Antagonists are conventionally presented as making moral choices less savory than those of protagonists. This condition is often used by an author to create conflict within a story. This is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Journal Of Pharmacology
The ''British Journal of Pharmacology'' is a biweekly peer-reviewed medical journal covering all aspects of experimental pharmacology. It is published for the British Pharmacological Society by Wiley-Blackwell. It was established in 1946 as the ''British Journal of Pharmacology and Chemotherapy''. The journal obtained its current title in 1968. The current editor-in-chief is Amrita Ahluwalia. Previous editors-in-chief include Ian McGrath, Humphrey Rang, Alan North, Phil Moore, Bill Large, and Tony Birmingham. A sister journal, also published for the British Pharmacological Society by Wiley-Blackwell is the '' British Journal of Clinical Pharmacology''. The journal publishes research papers, review articles, commentaries and correspondence in all fields of pharmacology. It also publishes themed issues, as well as supplements. ''The Concise Guide to PHARMACOLOGY'' The ''Concise Guide to PHARMACOLOGY'' is a supplement of the ''British Journal of Pharmacology'', replacing the "Guide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-1 Adrenergic Receptor
alpha-1 (α1) adrenergic receptors are G protein-coupled receptors (GPCRs) associated with the Gq heterotrimeric G protein. α1-adrenergic receptors are subdivided into three highly homologous subtypes, i.e., α1A-, α1B-, and α1D-adrenergic receptor subtypes. There is no α1C receptor. At one time, there was a subtype known as α1C, but it was found to be identical to the previously discovered α1A receptor subtype. To avoid confusion, naming was continued with the letter D. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α1-adrenergic receptors in the central and peripheral nervous systems. The crystal structure of the α1B-adrenergic receptor subtype has been determined in complex with the inverse agonist (+)-cyclazosin. Effects The α1-adrenergic receptor has several general functions in common with the α2-adrenergic receptor, but also has specific effects of its own. α1-receptors primarily mediate smooth muscle cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscarinic Acetylcholine Receptor M2
The muscarinic acetylcholine receptor M2, also known as the cholinergic receptor, muscarinic 2, is a muscarinic acetylcholine receptor that in humans is encoded by the ''CHRM2'' gene. Multiple alternatively spliced transcript variants have been described for this gene. Function Heart The M2 muscarinic receptors are located in the heart, where they act to slow the heart rate down to normal sinus rhythm after negative stimulatory actions of the parasympathetic nervous system, by slowing the speed of depolarization. They also reduce contractile forces of the atrial cardiac muscle, and reduce conduction velocity of the atrioventricular node (AV node). However, they have little effect on the contractile forces of the heart ventricle, ventricular muscle, slightly decreasing force. IQ A Dutch family study "a highly significant association" between the ''CHRM2'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscarinic Acetylcholine Receptor M3
The muscarinic acetylcholine receptor, also known as cholinergic/acetylcholine receptor M3, or the muscarinic 3, is a muscarinic acetylcholine receptor encoded by the human gene CHRM3. The M3 muscarinic receptors are located at many places in the body, e.g., smooth muscles, the endocrine glands, the exocrine glands, lungs, pancreas and the brain. In the CNS, they induce emesis. Muscarinic M3 receptors are expressed in regions of the brain that regulate insulin homeostasis, such as the hypothalamus and dorsal vagal complex of the brainstem. These receptors are highly expressed on pancreatic beta cells and are critical regulators of glucose homoestasis by modulating insulin secretion. In general, they cause smooth muscle contraction and increased glandular secretions. They are unresponsive to PTX and CTX. Mechanism Like the M1 muscarinic receptor, M3 receptors are coupled to G proteins of class Gq, which upregulate phospholipase C and, therefore, inositol trisphosphate and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine H1 Receptor
The H1 receptor is a histamine receptor belonging to the family of rhodopsin-like G-protein-coupled receptors. This receptor is activated by the biogenic amine histamine. It is expressed in smooth muscles, on vascular endothelial cells, in the heart, and in the central nervous system. The H1 receptor is linked to an intracellular G-protein (Gq) that activates phospholipase C and the inositol triphosphate (IP3) signalling pathway. Antihistamines, which act on this receptor, are used as anti-allergy drugs. The crystal structure of the receptor has been determined (shown on the right/below) and used to discover new histamine H1 receptor ligands in structure-based virtual screening studies. Function The expression of NF-κB, the transcription factor that regulates inflammatory processes, is promoted by the constitutive activity of the H1 receptor as well as by agonists that bind at the receptor. H1-antihistamines have been shown to attenuate NF-κB expression and mitigate certain in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine A1 Receptor
The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand. Biochemistry A1 receptors are implicated in sleep promotion by inhibiting wake-promoting cholinergic neurons in the basal forebrain. A1 receptors are also present in smooth muscle throughout the vascular system. The adenosine A1 receptor has been found to be ubiquitous throughout the entire body. Signalling Activation of the adenosine A1 receptor by an agonist causes binding of Gi1/2/3 or Go protein. Binding of Gi1/2/3 causes an inhibition of adenylate cyclase and, therefore, a decrease in the cAMP concentration. An increase of the inositol triphosphate/diacylglycerol concentration is caused by an activation of phospholipase C, whereas the elevated levels of arachidonic acid are mediated by DAG lipase, which cleaves DAG to form arachidonic acid. Several types of potassium channels are activated but N-, P-, and Q-type calcium channels are in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
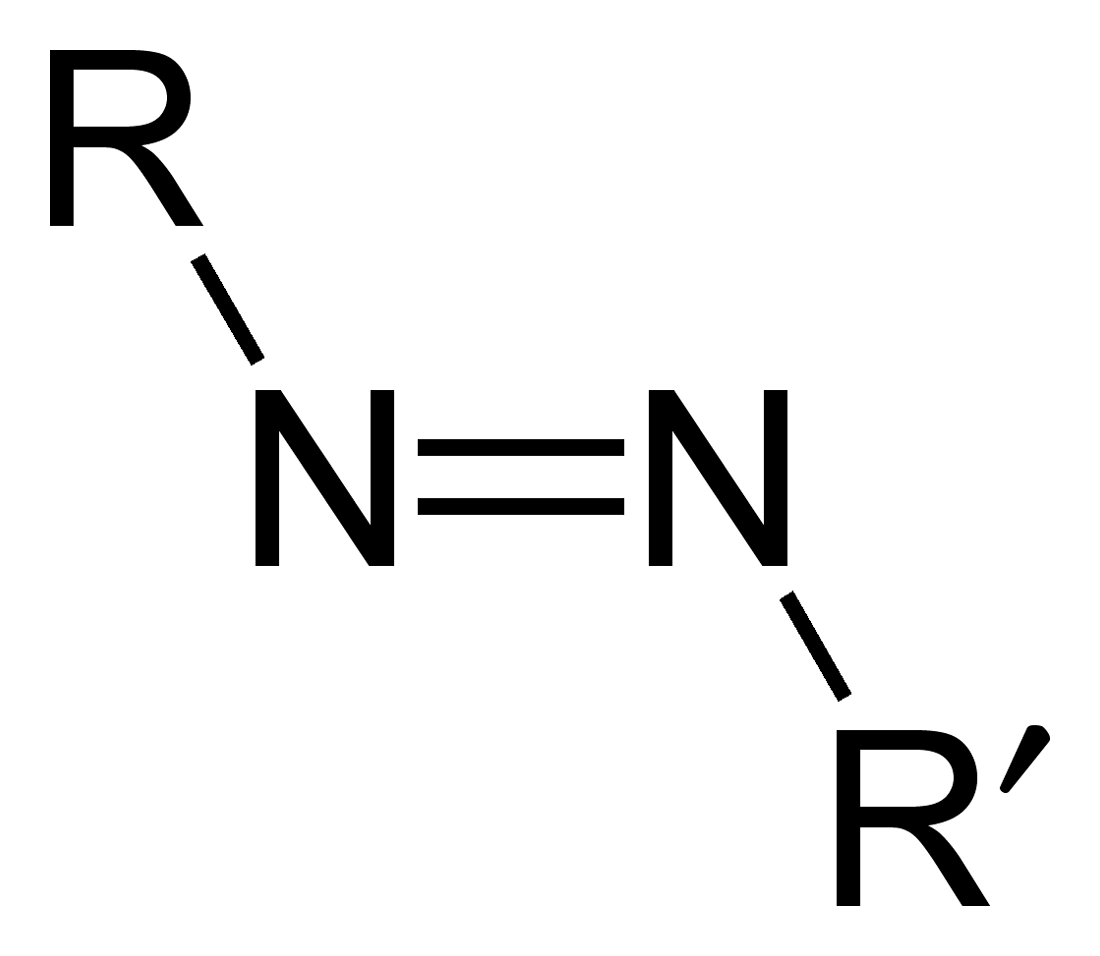
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphates
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridines
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |