|
PHIST
The ''Plasmodium'' helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing ''Plasmodium'' species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, but the identification of this part associated with a DnaJ domain in ''P. falciparum'' RESA (named after the ring stage of the parasite) has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc. The PHIST proteins are exported to the cytoplasm of the infected erythrocyte. The human malaria parasites ''P. falciparum'' and '' P. vivax'' have shown a lineage-specific expansion of proteins with this domain. Of the two PHIST genes in the mouse parasite '' P. berghei'', only one is required for infection. The PHIST domain folds into three long helices (forming a bundle) and two smaller N-te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PfEMP1
''Plasmodium falciparum'' erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells (RBCs or erythrocytes) that are infected by the malarial parasite ''Plasmodium falciparum''. PfEMP1 is synthesized during the parasite's blood stage (erythrocytic schizogony) inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with ''P. falciparum''. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with Family (biology), family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar protein structure, three-dimensional structures, functions, and significant Sequence homology, sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
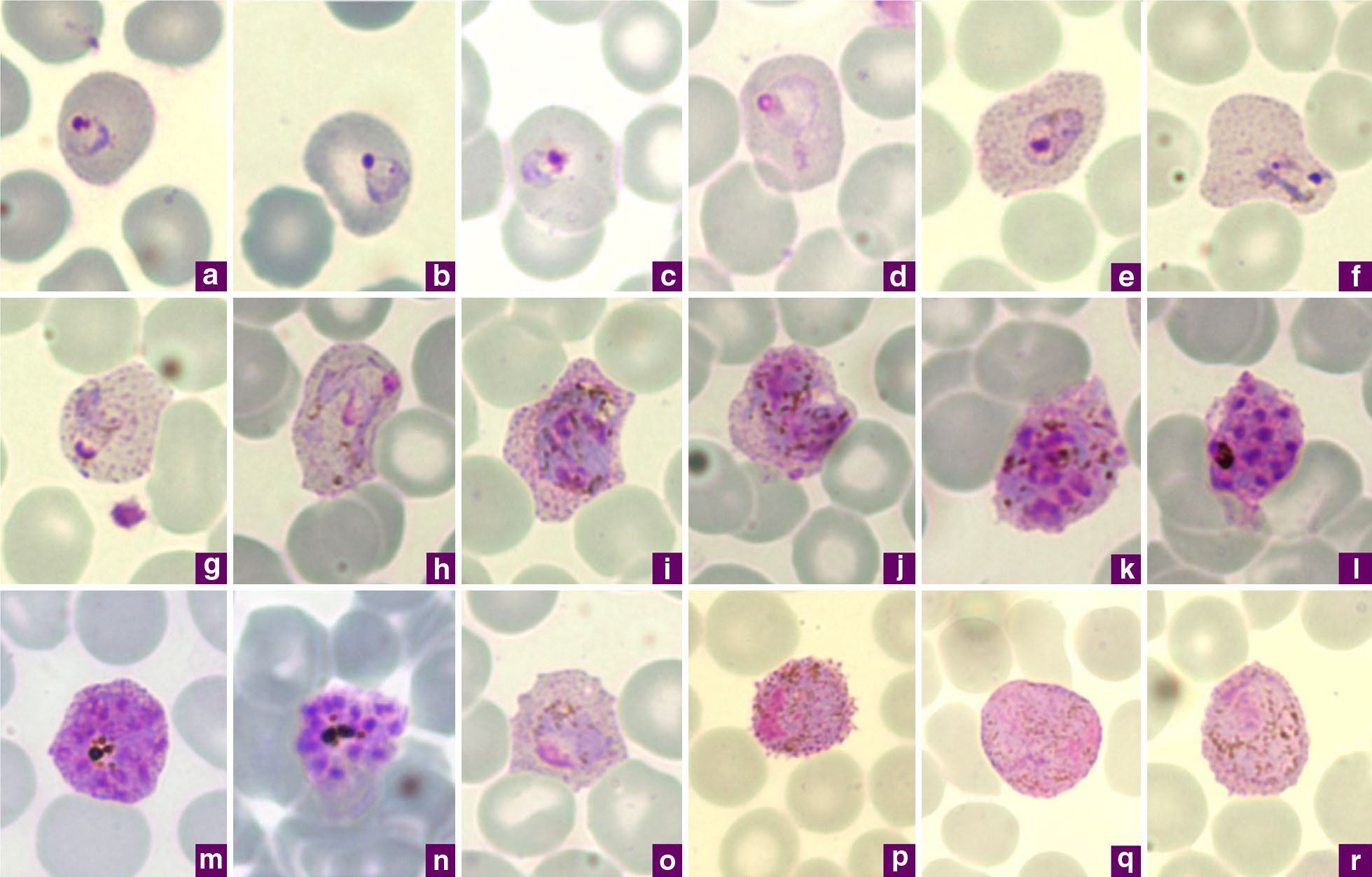
Malaria
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin ten to fifteen days after being bitten by an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria. Malaria is caused by single-celled microorganisms of the ''Plasmodium'' group. It is spread exclusively through bites of infected ''Anopheles'' mosquitoes. The mosquito bite introduces the parasites from the mosquito's saliva into a person's blood. The parasites travel to the liver where they mature and reproduce. Five species of ''Plasmodium'' can infect and be spread by h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmodium
''Plasmodium'' is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of ''Plasmodium'' species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue (often the liver) before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect (mosquitoes in majority cases), continuing the life cycle. ''Plasmodium'' is a member of the phylum Apicomplexa, a large group of parasitic eukaryotes. Within Apicomplexa, ''Plasmodium'' is in the order Haemosporida and family Plasmodiidae. Over 200 species of ''Plasmodium'' have been described, many of which have been subdivided into 14 subgenera based on parasite morphology and host range. Evolutionary relationships among different ''Pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DnaJ
In molecular biology, chaperone DnaJ, also known as Hsp40 (heat shock protein 40 kD), is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans. Function Molecular chaperones are a diverse family of proteins that function to protect proteins from irreversible aggregation during synthesis and in times of cellular stress. The bacterial molecular chaperone DnaK is an enzyme that couples cycles of ATP binding, hydrolysis, and ADP release by an N-terminal ATP-hydrolyzing domain to cycles of sequestration and release of unfolded proteins by a C-terminal substrate binding domain. Dimeric GrpE is the co-chaperone for DnaK, and acts as a nucleotide exchange factor, stimulating the rate of ADP release 5000-fold. DnaK is itself a weak ATPase; ATP hydrolysis by DnaK is stimulated by its interaction with another co-chaperone, DnaJ. Thus the co-chaperones DnaJ and GrpE are capable of tightly regulating the nucleotide-bound and substrate-bound st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmodium Vivax
''Plasmodium vivax'' is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than ''Plasmodium falciparum'', the deadliest of the five human malaria parasites, ''P. vivax'' malaria infections can lead to severe disease and death, often due to splenomegaly (a pathologically enlarged spleen). ''P. vivax'' is carried by the female ''Anopheles'' mosquito; the males do not bite. Health Epidemiology ''Plasmodium vivax'' is found mainly in Asia, Latin America, and in some parts of Africa. ''P. vivax'' is believed to have originated in Asia, but recent studies have shown that wild chimpanzees and gorillas throughout central Africa are endemically infected with parasites that are closely related to human ''P. vivax.'' These findings indicate that human P. vivax is of African origin. ''Plasmodium vivax'' accounts for 65% of malaria cases in Asia and South America. Unlike ''Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmodium Berghei
''Plasmodium berghei'' is a species in the genus ''Plasmodium'' subgenus ''Vinckeia''. It is a protozoan parasite that causes malaria in certain rodents. Originally, isolated from thicket rats in Central Africa, ''P. berghei'' is one of four ''Plasmodium'' species that have been described in African murine rodents, the others being '' P. chabaudi'', '' P. vinckei'', and '' P. yoelii''. Due to its ability to infect rodents and relative ease of genetic engineering, ''P. berghei'' is a popular model organism for the study of human malaria. Biology Like all malaria parasites of mammals, including the four human malaria parasites, ''P. berghei'' is transmitted by ''Anopheles'' mosquitoes and it infects the liver after being injected into the bloodstream by a bite of an infected female mosquito. After a short period (a few days) of development and multiplication, these parasites leave the liver and invade erythrocytes (red blood cells). The multiplication of the parasite in the bloo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helix Bundle
A helix bundle is a small protein fold composed of several alpha helices that are usually nearly parallel or antiparallel to each other. Three-helix bundles Three-helix bundles are among the smallest and fastest known cooperatively folding structural domains. The three-helix bundle in the villin headpiece domain is only 36 amino acids long and is a common subject of study in molecular dynamics simulations because its microsecond-scale folding time is within the timescales accessible to simulation. The 40-residue HIV accessory protein has a very similar fold and has also been the subject of extensive study. There is no general sequence motif associated with three-helix bundles, so they cannot necessarily be predicted from sequence alone. Three-helix bundles often occur in actin-binding proteins and in DNA-binding proteins. Four-helix bundles Four-helix bundles typically consist of four helices packed in a coiled-coil arrangement with a sterically close-packed hydrophobic core in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrin
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antigens
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. The term ''antigen'' originally referred to a substance that is an antibody generator. Antigens can be proteins, peptides (amino acid chains), polysaccharides (chains of monosaccharides/simple sugars), lipids, or nucleic acids. Antigens are recognized by antigen receptors, including antibodies and T-cell receptors. Diverse antigen receptors are made by cells of the immune system so that each cell has a specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize that antigen are activated and expanded, a process known as clonal selection. In most cases, an antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cross-react and bind more than one antigen. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |