|
PCP Site 2
PCP site 2 is a binding site that was identified as a high-affinity target for phencyclidine (PCP), an anesthetic and dissociative hallucinogen that acts primarily as an NMDA receptor antagonist. The site is distinct from the PCP binding site on the NMDA receptor (otherwise known as PCP site 1) and the common/main sites on the monoamine transporters (, , ). It is associated with monoamine reuptake inhibition, and it has been suggested that the site may be an allosteric/regulatory site of the monoamine transporters. RTI-4793-14 (HBMP), a ligand with high affinity for the PCP site 2 and high selectivity for this site over the PCP site 1, has been developed. Similarly to PCP, RTI-4793-1 inhibits monoamine reuptake with moderate potency, but unlike PCP, has very low potency as an NMDA receptor antagonist. It shows a profile of a serotonin–norepinephrine–dopamine reuptake inhibitor A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phencyclidine Structure
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and violent behavior. As a recreational drug, it is typically smoked, but may be taken by mouth, snorted, or injected. It may also be mixed with cannabis or tobacco. Adverse effects may include seizures, coma, addiction, and an increased risk of suicide. Flashbacks may occur despite stopping usage. Chemically, PCP is a member of the arylcyclohexylamine class, and pharmacologically, it is a dissociative anesthetic. PCP works primarily as an NMDA receptor antagonist. PCP is most commonly used in the United States. While usage peaked in the US in the 1970s, between 2005 and 2011 an increase in visits to emergency departments as a result of the drug occurred. As of 2017 in the United States, about 1% of people in Twelfth g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
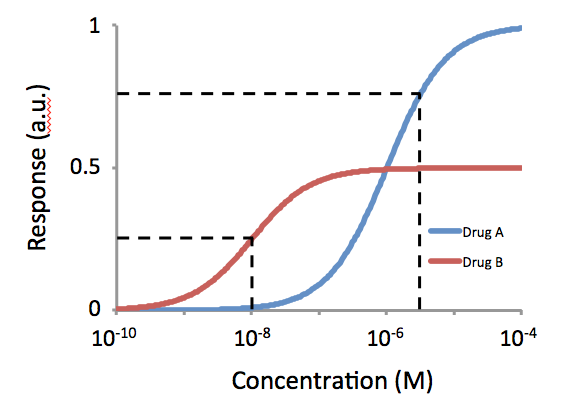
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketamine
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a recreational drug. It is one of the safest anesthetics, as, in contrast with opiates, ether, and propofol, it suppresses neither respiration nor heart rate. Ketamine is also simple to administer and highly tolerable compared to drugs with similar effects which are flammable, irritating, or even explosive. Ketamine is a novel compound, derived from PCP, created in pursuit of a safer anesthetic with similar characteristics. Ketamine is also used for acute pain management. At anesthetic doses, ketamine induces a state of "dissociative anesthesia", a trance-like state providing pain relief, sedation, and amnesia. The distinguishing features of ketamine anesthesia are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation. At lower, sub-anesthetic doses, ketamine is a promising agent for pain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tiletamine
Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist. It is related chemically to ketamine. Tiletamine hydrochloride exists as odorless white crystals. It is used in veterinary medicine in the combination product Telazol (tiletamine/zolazepam, 50 mg/ml of each in 5 ml vial) as an injectable anesthetic for use in cats and dogs. It is sometimes used in combination with xylazine (Rompun) to chemically immobilize large mammals such as polar bears and wood bison. Telazol is the only commercially available tiletamine product in the United States. It is contraindicated in patients of an ASA score of III or greater and in animals with CNS signs, hyperthyroidism, cardiac disease, pancreatic or renal disease, pregnancy, glaucoma, or penetrating eye injuries. Recreational use of telazol has been documented. Animal studies have also shown that tiletamine produces rewarding and reinforcing effects. Tiletamine products are classified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dexoxadrol
Dexoxadrol (Dioxadrol) is a dissociative anaesthetic drug which has been found to be an NMDA antagonist and produces similar effects to PCP in animals. Dexoxadrol, along with another related drug etoxadrol, were developed as analgesics for use in humans, but development was discontinued after patients reported side effects such as nightmares A nightmare, also known as a bad dream, Retrieved 11 July 2016. is an unpleasant dream that can cause a strong emotional response from the mind, typically fear but also despair, anxiety or great sadness. The dream may contain situations of d ... and hallucinations. References Dissociative drugs Piperidines Dioxolanes Abandoned drugs {{hallucinogen-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenocyclidine
Tenocyclidine (TCP) is a dissociative anesthetic with psychostimulant effects. It was discovered by a team at Parke-Davis in the late 1950s. Heterocyclic compounds and methods for producing the same It is similar in effects to phencyclidine (PCP) but is considerably more potent. TCP has slightly different binding properties to PCP, with more affinity for the NMDA receptors, but less affinity for the sigma receptors. Because of its high affinity for the PCP site of the NMDA receptor complex, the 3H radiolabelled form of TCP is widely used in research into NMDA receptors. TCP acts primarily as an NMDA receptor antagonist which blocks the activity of the NMDA receptor, however its increased psychostimulant effects compared to PCP suggests it also has relatively greater activity as a dopamine reuptake inhibitor (DRI). Due to its similarity in effects to PCP, TCP was placed into the Schedule I list of illegal drugs in the 1970s, although it was only briefly used in the 1970s and 1980 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMDA
''N''-methyl--aspartic acid or ''N''-methyl--aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike glutamate, NMDA only binds to and regulates the NMDA receptor and has no effect on other glutamate receptors (such as those for AMPA and kainate). NMDA receptors are particularly important when they become overactive during, for example, withdrawal from alcohol as this causes symptoms such as agitation and, sometimes, epileptiform seizures. Biological function In 1962, J.C. Watkins reported synthesizing NMDA, an isomer of the previously know''N''-Methyl--aspartic-acid (PubChem ID 4376) NMDA is a water-soluble -alpha-amino acid — an aspartic acid derivative with an ''N''-methyl substituent and - configuration — found across Animalia from lancelets to mammals. At homeostatic levels NMDA plays an essential role as a neurotransmitter and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nisoxetine
Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects. Researchers have attempted to use a carbon- labeled form of nisoxetine for positron emission tomography (PET) imaging of the norepinephrine transporter (NET), with little success. However, it seems that tritium labeled nisoxetine (3H-nisoxetine, 3H-NIS) is a useful radioligand for labeling norepinephrine uptake sites ''in vitro'', which nisoxetine and other antagonists for NET are able to inhibit. History In treating depression, it was theorized that substances that co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WIN-35428
(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane (β-CFT, WIN 35,428) is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the Armstrong's acid, naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported. Uses CFT was first reported by Clarke and co-workers in 1973. This drug is known to function as a "positive reinforcer" (although it is less likely to be self-administered by rhesus monkeys than cocaine). Tritiated CFT is frequently used to map binding of novel ligands to the Dopamine active transporter, DAT, although the drug also has some Serotonin transporter, SERT affinity. Isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor (chemistry), precursor chemical, L-DOPA, which is biosynthesis, synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopaminergic pathway, dopamine pathways, one of which plays a major role in the motivational component of reward system, reward-motivated behavior. The anticipa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indatraline
Indatraline (Lu 19-005) is a non- selective monoamine transporter inhibitor shown to block the reuptake of dopamine, norepinephrine, and serotonin, with effects similar to those of cocaine. The effects have been shown to have a slower onset and longer duration than cocaine, suggesting that the compound may, along with similar compounds, be used for the treatment of cocaine addiction. LU 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments. Superposition should make it possible to see there is at least a fundamental relationship between the pharmacophore of indatraline and various phenyltropanes. Methylation If indatraline is ''N''-alkylated at the amino group, it is possible to slow the onset of action so that it is not until ''N''-demethylation occurs that the molecules become active. ''N''-methylindatraline has a much longer duration than indatraline because norindatraline is inactive, whereas demethylating ''N''-methylindatraline doe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)