|
Ostwald's Rule
In materials science, Ostwald's rule or Ostwald's step rule, conceived by Wilhelm Ostwald, describes the formation of polymorphs. The rule states that usually the less stable polymorph crystallizes first. Unstable polymorphs more closely resemble the state in solution, and thus are kinetically advantaged. From hot water, metastable, fibrous crystals of benzamide appear first, later to spontaneously convert to the more stable rhombic polymorph. Another example is magnesium carbonate, which more readily forms dolomite. A dramatic example is phosphorus, which upon sublimation first forms the less stable white phosphorus, which only slowly polymerizes to the red allotrope. This is notably the case for the anatase polymorph of titanium dioxide, which having a lower surface energy In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm Ostwald
Friedrich Wilhelm Ostwald (; 4 April 1932) was a Baltic German chemist and German philosophy, philosopher. Ostwald is credited with being one of the founders of the field of physical chemistry, with Jacobus Henricus van 't Hoff, Walther Nernst, and Svante Arrhenius. He received the Nobel Prize in Chemistry in 1909 for his scientific contributions to the fields of catalysis, chemical equilibria and Reaction velocity, reaction velocities. Following his 1906 retirement from academic life, Ostwald became much involved in philosophy, art, and politics. He made significant contributions to each of these fields. He has been described as a polymath. Early life and education Ostwald was born ethnically Baltic German in Riga, Russian Empire (now Latvia) to cooper (profession), master-cooper Gottfried Wilhelm Ostwald (1824–1903) and Elisabeth Leuckel (1824–1903). He was the middle child of three, born after Eugen (1851–1932) and before Gottfried (1855–1918). Ostwald developed an i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
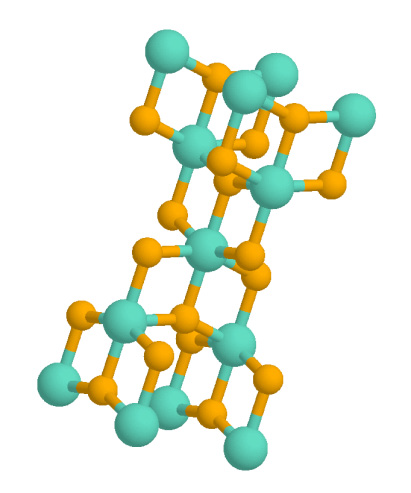
Dolomite (mineral)
Dolomite () is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite. An alternative name sometimes used for the dolomitic rock type is dolostone. History As stated by Nicolas-Théodore de Saussure the mineral dolomite was probably first described by Carl Linnaeus in 1768. In 1791, it was described as a rock by the French naturalist and geologist Déodat Gratet de Dolomieu (1750–1801), first in buildings of the old city of Rome, and later as samples collected in the mountains now known as the Dolomite Alps of northern Italy. Nicolas-Théodore de Saussure first named the mineral (after Dolomieu) in March 1792. Properties The mineral dolomite crystallizes in the trigonal-rhombohedral system. It forms white, tan, gray, or pink crystals. Dolomite is a double carbonate, having an alternating structural arrangement of calcium and magnesium ions. Unless it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the " Morning Star", the planet Venus. The term '' phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in a different manner. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a cubic lattice of tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for compounds. The more general term, used for any compound, is polymorphism, although its use is usually restricted to solid materials such as crystals. Allotropy refers only to different forms of an element within the same physical phase (the state of matter, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble to water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structure of rutile is tetragonal in symmetry whereas anatase and brookite are orthorhombic. The oxygen substructures are all slight distort ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material (the atoms on the surface have more energy compared with the atoms in the bulk), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding at the two surfaces. Cutting a solid body into pieces disrupts its bonds and increases the surface area, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. History Early writing on mineralogy, especially on gemstones, comes from ancient Babylonia, the ancient Greco-Roman world, ancient and medieval China, and Sanskrit texts from ancient India and the ancient Islamic world. Books on the subject included the ''Naturalis Historia'' of Pliny the Elder, which not only described many different minerals but also explained many of their properties, and Kitab al Jawahir (Book of Precious Stones) by Persian scientist Al-Biruni. The German Renaissance specialist Georgius Agricola wrote works such as '' De re metallica'' (''On Metals'', 1556) and ''De Natura Fossilium'' ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gemology
Gemology or gemmology is the science dealing with natural and artificial gemstone materials. It is a geoscience and a branch of mineralogy. Some jewelers (and many non-jewelers) are academically trained gemologists and are qualified to identify and evaluate gems. History Rudimentary education in gemology for jewellers and gemologists began in the nineteenth century, but the first qualifications were instigated after the National Association of Goldsmiths of Great Britain (NAG) set up a Gemmological Committee for this purpose in 1908. This committee matured into the Gemmological Association of Great Britain (also known as Gem-A), now an educational charity and accredited awarding body with its courses taught worldwide. The first US graduate of Gem-A's diploma course, in 1929, was Robert Shipley, who later established both the Gemological Institute of America and the American Gem Society. There are now several professional schools and associations of gemologists and certificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |