|
Organoruthenium
Organoruthenium chemistry is the chemistry Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ... of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in Group 8 element, group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl. In its organometallic compounds, ruthenium is known to adopt oxidation states from -2 ([Ru(CO)4]2−) to +6 ([RuN(Me)4]−). Most common are those in the 2+ oxidation state, as illustrated below. File:G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triruthenium Dodecacarbonyl
Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds. Structure and synthesis The cluster has ''D3h'' symmetry, consisting of an equilateral triangle of Ru atoms, each of which bears two axial and two equatorial CO ligands. Os3(CO)12 has the same structure, whereas Fe3(CO)12 is different, with two bridging CO ligands, resulting in C2v symmetry. Ru3(CO)12 is prepared by treating solutions of ruthenium trichloride with carbon monoxide in the presence of a base. Dichlororuthenium tricarbonyl dimer is an intermediate. The stoichiometry of the reaction is uncertain, one possibility being the following: :6 RuCl3 + 33 CO + 18 CH3OH → 2 Ru3(CO)12 + 9 CO(OCH3)2 + 18 HCl Reactions The chemical properties of Ru3(CO)12 have been widely studied, and the cluster ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grubbs Catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis. First-generation catalyst In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was reported in 1992. It was prepared from RuCl2(PPh3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russian Empire, Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009Summary. Ruthenium platinum.matthey.com, p. 9 (2009) to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylcyclopentadienyl Ruthenium Dichloride Dimer
Pentamethylcyclopentadienyl ruthenium dichloride is an organoruthenium chemistry with the formula C5(CH3)5)RuCl2sub>2, commonly abbreviated p*RuCl2sub>2. This brown paramagnetic solid is a reagent in organometallic chemistry. It is an unusual example of a compound that exists as isomers that differ in the intermetallic separation, a difference that is manifested in a number of physical properties. Preparation, structure, reactions The compound has C2h symmetry, with each metal atom having pseudo-octahedral geometry. In the crystal structure, two isomers are observed in the unit cell, one with a 2.93 Å ruthenium–ruthenium bond and the other with a long internuclear distance of 3.75 Å. The former isomer is diamagnetic, and the latter is magnetic. It is prepared by the reaction of hydrated ruthenium trichloride with pentamethylcyclopentadiene. :2 Cp*H + 2 RuCl3·3H2O → p*RuCl2sub>2 + 2 HCl + 6 H2O The reaction is accompanied by formation of decamethylruthenocene. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Reflection symmetry, Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone. Preparation Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium was first reported in 1969 when it was prepared by reacting dichlorotris(triphenylphosphine)ruthenium(II) with cyclopentadiene. :Dichlorotris(triphenylphosphine)ruthenium(II), RuCl2(PPh3)3 + C5H6 → RuCl(PPh3)3(C5H5) + HCl It is prepared by heating a mixture of ruthenium(III) chloride, triphenylphosphine, and cyclopentadiene in ethanol. Reactions Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium(II) undergoes a variety of reactions often by involving substitution of the chloride. With phenylacetylene it gives the phen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shvo Catalyst
The Shvo catalyst is an organoruthenium compound that catalyzes the hydrogenation of polar functional groups including aldehydes, ketones and imines. The compound is of academic interest as an early example of a catalyst for transfer hydrogenation that operates by an "outer sphere mechanism". Related derivatives are known where p-tolyl replaces some of the phenyl groups. Shvo's catalyst represents a subset of homogeneous hydrogenation catalysts that involves both metal and ligand in its mechanism. Synthesis and structure The catalyst is named after Youval Shvo, who uncovered it through studies on the effect of diphenylacetylene on the catalytic properties of triruthenium dodecacarbonyl. The reaction of diphenylacetylene and Ru3(CO)12 gives the piano stool complex . Subsequent hydrogenation of this tricarbonyl affords Shvo's catalyst. Y. Blum, D. Reshef, and Y. Shvo. H-transfer catalysis with Ru3(CO)12. Tetrahedron Lett. 22(16) 1981, pp. 1541-1544. Blum, Y.; Shvo, Y. Isr. J. Chem. 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene Compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less stable tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes. Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of oxygen. Nomenclature and terminology The nomenclature of the metal carbonyls depends on the charge of the complex, the number and type of central atoms, and the number and type of ligands and their binding modes. They occur as neutral complexes, as positively-charged metal carbonyl cations or as negatively charged metal carbonylates. The carbon monoxide liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
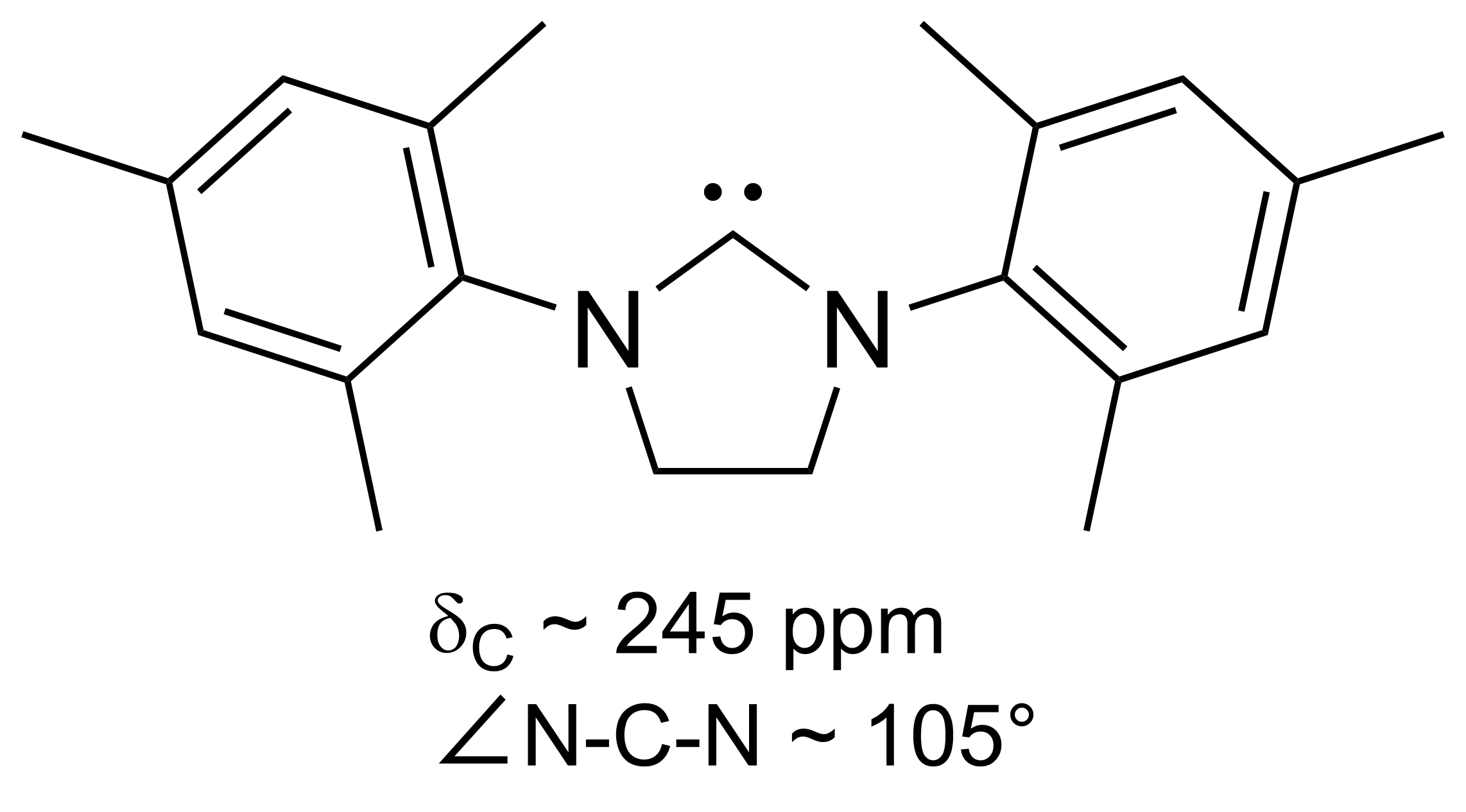
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Hydride
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2). Classes of metal hydrides Binary metal hydrides Many transition metals form compounds with hydrogen, called binary hydrides: binary, because these compounds contain only two elements, and hydride, because the hydrogenic ligand is assumed to have hydridic (H−-like) character. These compounds are invariably insoluble in all solvents, reflecting their polymeric structures. They often exhibit metal-like electrical conductivity. Many are nonstoichiometric compounds. Electropositive metals ( Ti, Zr, Hf, Zn) and some other metals form hydrides with the stoichiometry MH or sometimes MH2 (M = Ti, Zr, Hf, V, Zn). The b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2redox.png)