|
Omarigliptin
Omarigliptin (MK-3102) is a potent, long-acting oral antidiabetic drug of the DPP-4 inhibitor class used for once-weekly treatment of type 2 diabetes and currently under development by Merck & Co. It inhibits DPP-4 to increase incretin levels (GLP-1 and GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying and decreases blood glucose levels. History Marizev (omarigliptin) 25 mg and 12.5 mg tablets were approved by Japan's Pharmaceuticals and Medical Devices Agency (PMDA) on 28th Sept 2015. Japan was the first country to have approved omarigliptin. However Merck has announced that the company will not submit marketing application in the US and Europe. References See also * Dipeptidyl peptidase-4 inhibitor * Alogliptin * Linagliptin * Saxagliptin * Sitagliptin * Vildagliptin Vildagliptin, sold under the brand name Galvus and others, is an oral anti-hyperglycemic agent (anti-diabetic drug) of the dipeptidyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptidyl Peptidase-4 Inhibitor
Inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors or gliptins) are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2. The first agent of the class – sitagliptin – was approved by the FDA in 2006. Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels. A 2018 meta-analysis found no favorable effect of DPP-4 inhibitors on all-cause mortality, cardiovascular mortality, myocardial infarction or stroke in patients with type 2 diabetes. Examples Drugs belonging to this class are: * Sitagliptin (FDA approved 2006, marketed by Merck & Co. as Januvia) * Vildagliptin (EU approved 2007, marketed in the EU by Novartis as Galvus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptidyl Peptidase-4 Inhibitors
Inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors or gliptins) are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2. The first agent of the class – sitagliptin – was approved by the FDA in 2006. Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels. A 2018 meta-analysis found no favorable effect of DPP-4 inhibitors on all-cause mortality, cardiovascular mortality, myocardial infarction or stroke in patients with type 2 diabetes. Examples Drugs belonging to this class are: * Sitagliptin (FDA approved 2006, marketed by Merck & Co. as Januvia) * Vildagliptin (EU approved 2007, marketed in the EU by Novartis as Galvus) * S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DPP-4 Inhibitor
Inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors or gliptins) are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2. The first agent of the class – sitagliptin – was approved by the FDA in 2006. Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels. A 2018 meta-analysis found no favorable effect of DPP-4 inhibitors on all-cause mortality, cardiovascular mortality, myocardial infarction or stroke in patients with type 2 diabetes. Examples Drugs belonging to this class are: * Sitagliptin (FDA approved 2006, marketed by Merck & Co. as Januvia) * Vildagliptin (EU approved 2007, marketed in the EU by Novartis as Galvus) * S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antidiabetic Drug
Drugs used in diabetes treat diabetes mellitus by altering the glucose level in the blood. With the exceptions of insulin, most GLP receptor agonists ( liraglutide, exenatide, and others), and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of anti-diabetic drugs, and their selection depends on the nature of the diabetes, age and situation of the person, as well as other factors. Diabetes mellitus type 1 is a disease caused by the lack of insulin. Insulin must be used in type 1, which must be injected. Diabetes mellitus type 2 is a disease of insulin resistance by cells. Type 2 diabetes mellitus is the most common type of diabetes. Treatments include agents that (1) increase the amount of insulin secreted by the pancreas, (2) increase the sensitivity of target organs to insulin, (3) decrease the rate at which glucose is absorbed from the gastrointestinal tract, and (4) i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
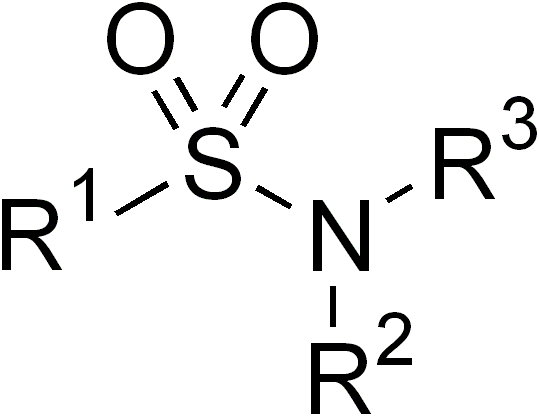
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vildagliptin
Vildagliptin, sold under the brand name Galvus and others, is an oral anti-hyperglycemic agent (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas. Vildagliptin has been shown to reduce hyperglycemia in type 2 diabetes mellitus. Combination with metformin The European Medicines Agency has also approved a combination of vildagliptin and metformin, vildagliptin/metformin (Eucreas by Novartis) as an oral treatment for type-2 diabetes. Adverse effects Adverse effects observed in clinical trials include nausea, hypoglycemia, tremor, headache and dizziness. Rare cases of hepatoxicity have been reported. There have been case reports of pancreatitis associated with DPP-4 inhibitors. A group at UCLA reported increased ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sitagliptin
Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes. In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available in the fixed-dose combination medication sitagliptin/metformin (Janumet, Janumet XR). Common side effects include headaches, swelling of the legs, and upper respiratory tract infections. Serious side effects may include angioedema, low blood sugar, kidney problems, pancreatitis, and joint pain. Whether use in pregnancy or breastfeeding is safe is unclear. It is in the dipeptidyl peptidase-4 (DPP-4) inhibitor class and works by increasing the production of insulin and decreasing the production of glucagon by the pancreas. Sitagliptin was developed by Merck & Co. and approved for medical use in the United States in 2006. In 2020, it was the 74th most commonly prescribed medication in the United States, with more than 9million ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saxagliptin
Saxagliptin, sold under the brand name Onglyza, is an oral hypoglycemic (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug. In April 2016, the U.S. FDA added a warning about increased risk of heart failure. This was based on data in an article that concluded "DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased. Although saxagliptin improves glycemic control, other approaches are necessary to reduce cardiovascular risk in patients with diabetes." Medical uses Saxagliptin is used as monotherapy or in combination with other drugs for the treatment of type 2 diabetes. It does not appear to decrease the risk of heart attacks or strokes. It increases the risk of hospitaliza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linagliptin
Linagliptin, sold under the brand name Tradjenta among others, is a medication used to treat type 2 diabetes (but not type 1) in conjunction with exercise and diet. It is generally less preferred than metformin and sulfonylureas as an initial treatment. It is taken by mouth. Common side effects include inflammation of the nose and throat. Serious side effects may include angioedema, pancreatitis, joint pain. Use in pregnancy and breastfeeding is not recommended. Linagliptin is a dipeptidyl peptidase-4 inhibitor that works by increasing the production of insulin and decreasing the production of glucagon by the pancreas. Linagliptin was approved for medical use in the United States, Japan, the European Union, Canada, and Australia in 2011. In 2020, it was the 293rd most commonly prescribed medication in the United States, with more than 1million prescriptions. From August 2021 linagliptin became available as a generic medicine in the US. Medical uses Linagliptin is indicated as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alogliptin
Alogliptin, sold under the brand names Nesina and Vipidia,) is an oral anti-diabetic drug in the DPP-4 inhibitor (gliptin) class. Alogliptin does not decrease the risk of heart attack and stroke. Like other members of the gliptin class, it causes little or no weight gain, exhibits relatively little risk of hypoglycemia, and has relatively modest glucose-lowering activity. Alogliptin and other gliptins are commonly used in combination with metformin in people whose diabetes cannot adequately be controlled with metformin alone. In April 2016, the U.S. Food and Drug Administration (FDA) added a warning about increased risk of heart failure. It was developed by Syrrx, a company which was acquired by Takeda Pharmaceutical Company in 2005. In 2020, it was the 295th most commonly prescribed medication in the United States, with more than 1million prescriptions. Medical uses Alogliptin is a dipeptidyl peptidase-4 inhibitor that decreases blood sugar similar to the other. Side effects A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceuticals And Medical Devices Agency
The (PhMDA) is an Independent Administrative Institution responsible for ensuring the safety, efficacy and quality of pharmaceuticals and medical devices in Japan. It is similar in function to the Food and Drug Administration in the United States, the Medicines and Healthcare products Regulatory Agency in the United Kingdom or the Food and Drug Administration in the Philippines. The PhMDA has been eCTD compliant at least since December 2017. Tasks Among other things, the agency is tasked with the following: * Drug and medical device testing: ** Scientific review of market authorization applications based on Japanese pharmaceutical law ** Advice in clinical trials or in the preparation of dossiers for the registration procedure (New Drug Applications (NDA)) ** Inspection and conformity assessment of Good Clinical Practice (GCP), Good Laboratory Practice (GLP), and Good Practice Systems and Programs (GPSP) ** Auditing of manufacturers to ensure they conform to Good Manufacturin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |