|
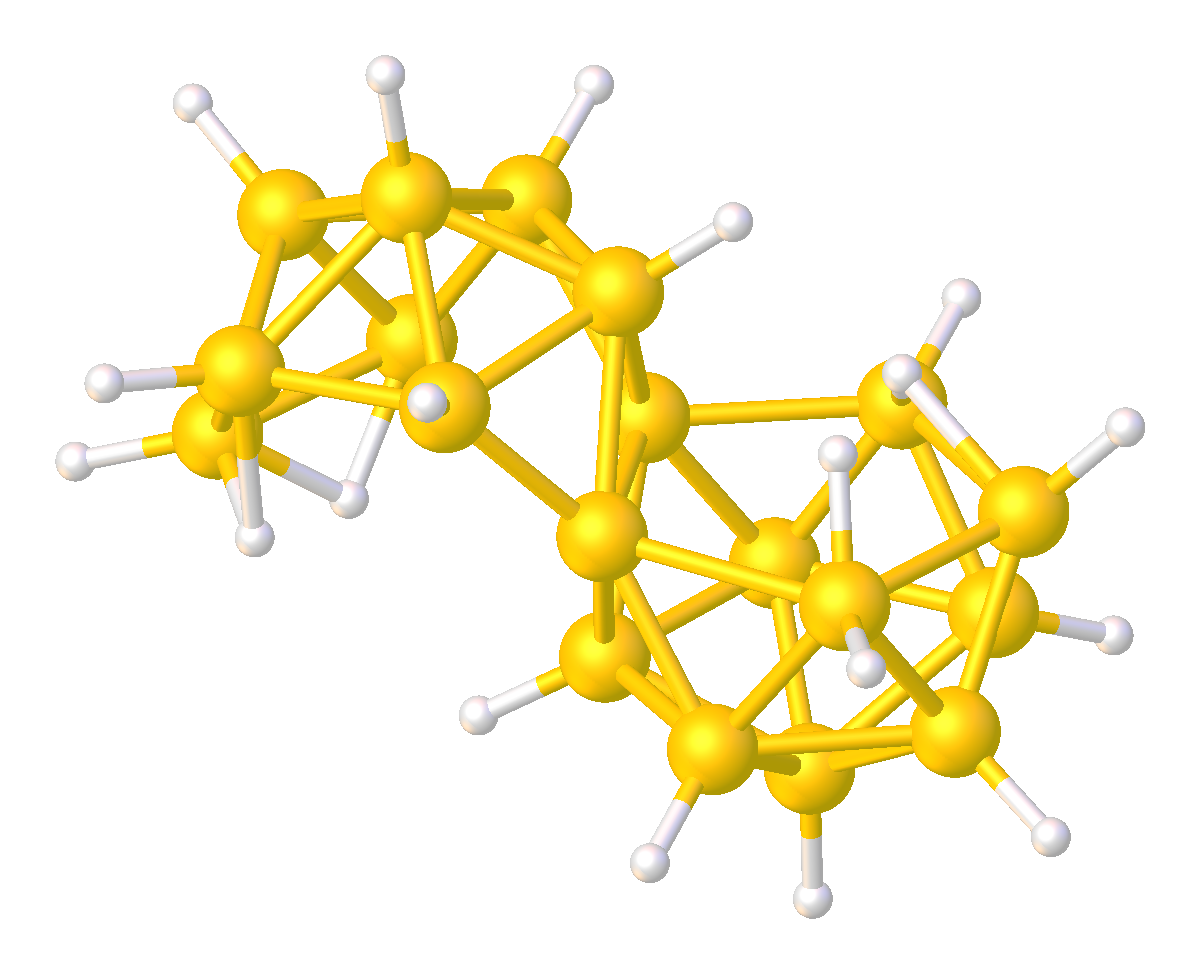
Octadecaborane
Octadecaborane is an inorganic compound, a boron hydride cluster with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest. Synthesis It is formed by oxidative degradation of B20H182− or by oxidative coupling of B9H12−. Structure Two isomers are known of octadecaborane, providing the first example of isomers in a boron-hydride cluster. The clusters are also of interest because the boron centers shared between the two subunits have an unusually high number of B-B interactions. The isomers consists of two B9H11 polyhedral subunits, each having a decaborane-like form, joined at a B–B edge. These two boron atoms are each coordinated to six others; this compound was the first one found to have such a high number of borons coordinated around a single boron center. There are two different geometric isomers of this compound, differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Hydride Cluster
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed. History The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization of decaborane. William Lipscomb was awarded the Nobel Prize in Chemistry in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers. The bonding of the clusters ushered in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is unknown or highly unstable. The term ''homodimer'' is used when the two subunits are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called Dissociation (chemistry), dissociation. When two oppositely-charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Danish chemist Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Many OH-containing molecules form dimers, e.g. the water dimer. Dimers that form based on w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhedra
In geometry, a polyhedron (: polyhedra or polyhedrons; ) is a three-dimensional figure with flat polygonal faces, straight edges and sharp corners or vertices. The term "polyhedron" may refer either to a solid figure or to its boundary surface. The terms solid polyhedron and polyhedral surface are commonly used to distinguish the two concepts. Also, the term ''polyhedron'' is often used to refer implicitly to the whole structure formed by a solid polyhedron, its polyhedral surface, its faces, its edges, and its vertices. There are many definitions of polyhedron. Nevertheless, the polyhedron is typically understood as a generalization of a two-dimensional polygon and a three-dimensional specialization of a polytope, a more general concept in any number of dimensions. Polyhedra have several general characteristics that include the number of faces, topological classification by Euler characteristic, duality, vertex figures, surface area, volume, interior lines, Dehn invari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decaborane
Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10 H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate. Handling, properties and structure The physical characteristics of decaborane(14) resemble those of naphthalene and anthracene, all three of which are volatile colorless solids. Sublimation is the common method of purification. Decaborane is highly flammable, and burns with a bright green flame like other boron hydrides. It is not sensitive to moist air, although it hydrolyzes in boiling water, releasing hydrogen and giving a solution of boric acid. It is soluble in cold water as well as a variety of non-polar and moderately polar solvents. In decaborane, the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superposed (not to be confused with superimposed) onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called '' enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, ''enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superposable mirror ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Resolution
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic mixture into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution. The use of chiral resolution to obtain enantiomerically pure compounds has the disadvantage of necessarily discarding at least half of the starting racemic mixture. Asymmetric synthesis of one of the enantiomers is one means of avoiding this waste. Crystallization of diastereomeric salts The most common method for chiral resolution involves conversion of the racemic mixture to a pair of diastereomeric derivatives by reacting them with chiral derivatizing agents, also known as chiral resolving agents. The derivatives which are then separated by conventional crystallization, and converted back to the enantiomers by removal of the resolving agent. The process can be laborious and depends on the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superposable. Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image. It is solely a relationship of chirality (chemistry), chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientation of a molecule as a whole or conformational isomerism, conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a ''racemic mixture'' or a ''racemate'', does not rotate light. Stereoisomers include both enantiomers and diastereomers. Diaste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |