|
Oct-1-en-3-one
Oct-1-en-3-one (CH2=CHC(=O)(CH2)4CH3), also known as 1-octen-3-one, is the odorant that is responsible for the typical "metallic" smell of metals and blood coming into contact with skin. Oct-1-en-3-one has a strong metallic mushroom-like odor with an odor detection threshold of 0.03–1.12 µg/m3 and it is the main compound responsible for the "smell of metal", followed by decanal (smell: orange skin, flowery) and nonanal (smell: tallowy, fruity). Oct-1-en-3-one is the degradative reduction product of the chemical reaction of skin lipid peroxides and Fe2+. Skin lipid peroxides are formed from skin lipid by oxidation, either enzymatically by lipoxygenases or by air oxygen. Oct-1-en-3-one is a ketone analog of the alkene 1-octene. Natural occurrences It is also produced by ''Uncinula necator'', a fungus that causes powdery mildew of grape. See also * Odorant An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a sm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aroma Compound
An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds. Generally, molecules meeting this specification have molecular weights of less than 310. Flavors affect both the sense of taste and smell, whereas fragrances affect only smell. Flavors tend to be naturally occurring, and the term ''fragrances'' may also apply to synthetic compounds, such as those used in cosmetics. Aroma compounds can naturally be found in various foods, such as fruits and their peels, wine, spices, floral scent, perfumes, fragranc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
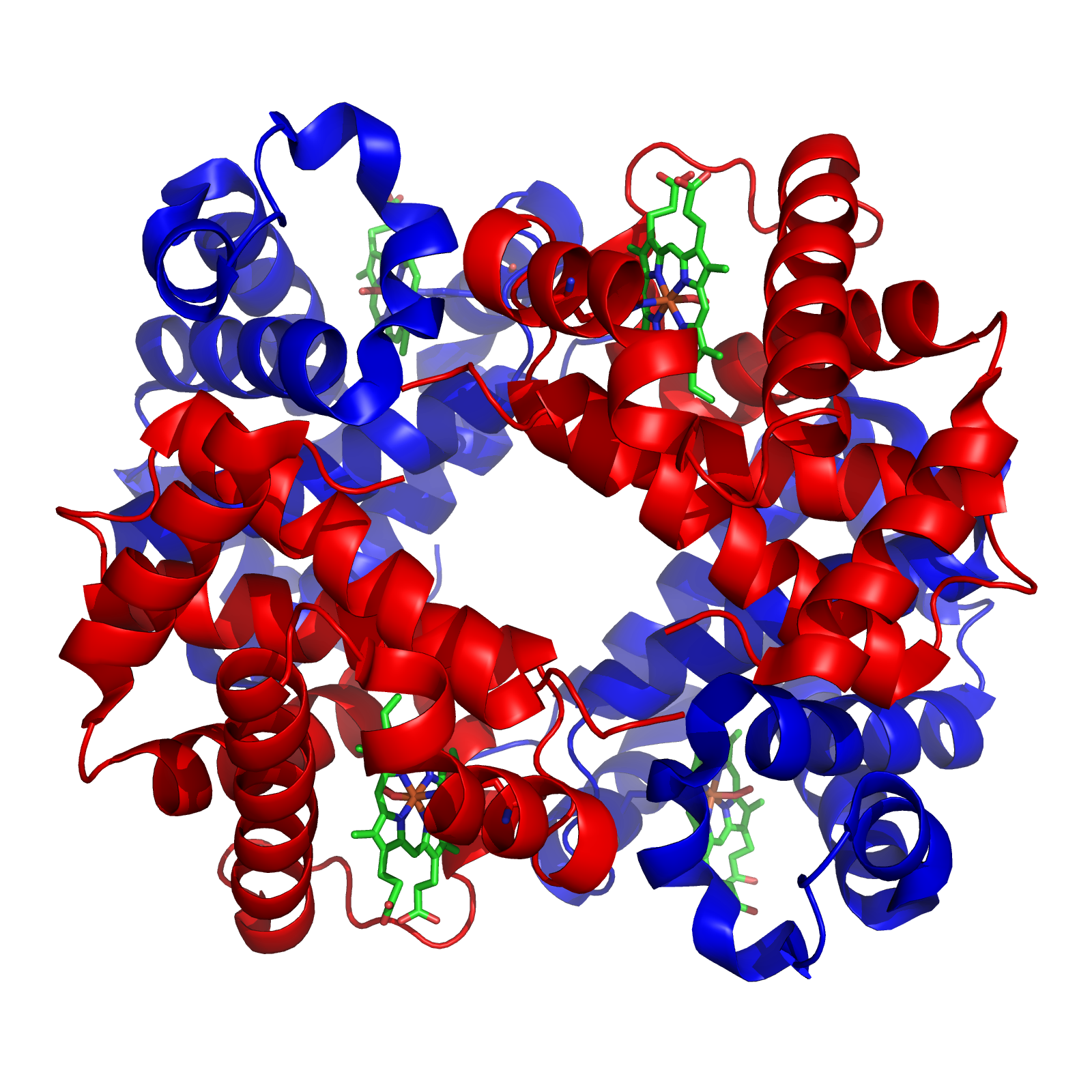
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in verte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uncinula Necator
''Uncinula necator'' (syn. ''Erysiphe necator'') is a fungus that causes powdery mildew of grape. It is a common pathogen of Vitis species, including the wine grape, ''Vitis vinifera''. The fungus is believed to have originated in North America. European varieties of ''Vitis vinifera'' are more or less susceptible to this fungus. ''Uncinula necator'' infects all green tissue on the grapevine, including leaves and young berries. It can cause crop loss and poor wine quality if untreated. The sexual stage of this pathogen requires free moisture to release ascospores from its cleistothecia in the spring. However, free moisture is not needed for secondary spread via conidia; high atmospheric humidity is sufficient. Its anamorph is called ''Oidium tuckeri''. It produces common odors such as 1-octen-3-one and (Z)- 1,5-octadien-3-one. This mildew can be treated with sulfur or fungicides; however resistance to several chemical classes such as Benomyl, the DMIs, and Strobilurins h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-octene
1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thus useful chemical properties. 1-Octene is one of the important linear alpha olefins in industry. It is a colourless liquid. Synthesis In industry, 1-octene is commonly manufactured by two main routes: oligomerization of ethylene and by Fischer–Tropsch synthesis followed by purification. Another route to 1-octene that has been used commercially on a small scale is dehydration of alcohols. Prior to the 1970s, 1-octene was also manufactured by thermal cracking of waxes, whereas linear internal octenes were also manufactured by chlorination/ dehydrochlorination of linear alkanes. There are five commercial processes that oligomerize ethylene to 1-octene. Four of these processes produce 1-octene as a part of a wide distribution of alpha-o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoxygenase
Lipoxygenases () are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells. The lipoxygenases are related to each other based upon their similar genetic structure and dioxygenation activity. However, one lipoxygenase, ALOXE3, while having a lipoxygenase genetic structure, possesses relatively little dioxygenation activity; rather its primary activity appears to be as an isomerase that catalyzes the conversion of hydroperoxy unsaturated fatty acids to their 1,5-epoxide, hydroxyl derivatives. Lipoxygenases are found in eukaryotes (plants, fungi, animals, protists); while the third domain of terrestrial life, the archaea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |