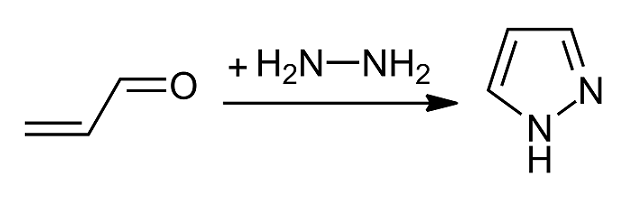|
O-1269
O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The ''N''-heptyl homolog O-1270 and the ''N''-propyl In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often ... homolog O-1399 also act as cannabinoid agonists with similar potency ''in vivo'', despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surinabant
Surinabant (SR147778) is a cannabinoid receptor type 1 antagonist developed by Sanofi-Aventis. It is being investigated as a potential treatment for nicotine addiction, to assist smoking cessation. It may also be developed as an anorectic drug to assist with weight loss, however there are already several CB1 antagonists or inverse agonists on the market or under development for this application, so surinabant is at present mainly being developed as an anti-smoking drug, with possible application in the treatment of other addictive disorders such as alcoholism. Other potential applications such as treatment of ADHD have also been proposed. A dose ranging study was done for smoking cessation in 2012; it did not improve success rate, but reduced weight gain. Inhibition of THC effects on heart rate was seen at 20 mg and 60 mg but not 5 mg. See also * Cannabinoid receptor antagonist * O-1269 O-1269 is a drug that is a diarylpyrazole derivative, related to potent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Antagonist
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid, is a non-competitive CB1/CB2 receptor antagonist. And Δ9-tetrahydrocannabivarin (THCV), a naturally occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rimonabant
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug that was first approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was the first drug approved in that class. History Rimonabant is a selective CB1 receptor blocker and was discovered and developed by Sanofi-Aventis; On 21 June 2006, the European Commission approved the sale of rimonabant in the then-25-member European Union as a prescription drug for use in conjunction with diet and exercise for patients with a body mass index (BMI) greater than 30 kg/m2, or patients with a BMI greater than 27 kg/m2 with associated risk factors, such as type 2 diabetes or dyslipidaemia. FroEMA index page/ref> It was first in its class to be approved anywhere in the world. Rimonabant was submitted to the Food and Dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Agonist
In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist , competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present. Some currently common drugs that have been classed as partial agonists at particular receptors include buspirone, aripiprazole, buprenorphine, nalmefene and norclozapine. Examples of ligands acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor
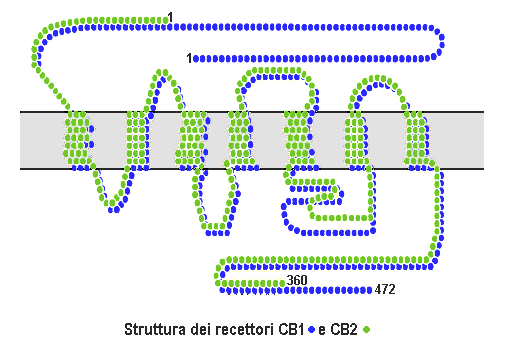
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sedation
Sedation is the reduction of irritability or agitation by administration of sedative drugs, generally to facilitate a medical procedure or diagnostic procedure. Examples of drugs which can be used for sedation include isoflurane, diethyl ether, propofol, etomidate, ketamine, pentobarbital, lorazepam and midazolam. Medical uses Sedation is typically used in minor surgical procedures such as endoscopy, vasectomy, or dentistry and for reconstructive surgery, some cosmetic surgeries, removal of wisdom teeth, or for high-anxiety patients. Sedation methods in dentistry include inhalation sedation (using nitrous oxide), oral sedation, and intravenous (IV) sedation. Inhalation sedation is also sometimes referred to as ''relative analgesia''. Sedation is also used extensively in the intensive care unit so that patients who are being ventilated tolerate having an endotracheal tube in their trachea. It can also be used during a long term brain EEG to help patient relax. Risks There are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fragments. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (chemistry)
In chemistry, homology is the appearance of homologues. A homologue (also spelled as homolog) is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene bridge −−, a peptide residue, etc. A homolog is a special case of an analog. Examples are alkanes and compounds with alkyl side chains of different length (the repeating unit being a methylene group -CH2-). Periodic table On the periodic table, homologous elements share many electrochemical properties and appear in the same group (column) of the table. For example, all noble gases are colorless, monatomic gases with very low reactivity. These similarities are due to similar structure in their outer shells of valence electrons. Mendeleev used the prefix eka- for an unknown element below a known one in the same group. See also * Homologous series * Analog Analog or analogue may refer to: Computing and electronics * Analog signal, in which information is enco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is a major constituent of temperate Cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea. Phytocannabinoids are multi-ring phenolic compounds structurally related to THC, but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |