|
Nowotny Phase
In inorganic chemistry, a Nowotny chimney ladder phase (NCL phase) is a particular intermetallic crystal structure found with certain binary compounds. NLC phases are generally tetragonal and are composed of two separate sublattices. The first is a tetragonal array of transition metal atoms, generally from group 4 element, group 4 through group 9 element, group 9 of the periodic table. Contained within this array of transition metal atoms is a second network of main group atoms, typically from Boron group, group 13 (boron group) or group 14 (carbon group). The transition metal atoms form a chimney with helical zigzag chain. The main-group elements form a ladder spiraling inside the transition metal helix. The phase is named after one of the early investigators H. Nowotny. Examples are RuGa2, Mn4Si7, Ru2Ge3, Ir3Ga5, Ir4Ge5 V17Ge31, Cr11Ge19, Mn11Si19, Mn15Si26, Mo9Ge16, Mo13Ge23, Rh10Ga17, and Rh17Ge22. In RuGa2 the ruthenium atoms in the chimney are separated by 329 pm. The galliu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Main Group
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements. The main group includes the elements (except hydrogen, which is sometimes not included) in groups 1 and 2 ( s-block), and groups 13 to 18 ( p-block). The s-block elements are primarily characterised by one main oxidation state, and the p-block elements, when they have multiple oxidation states, often have common oxidation states separated by two units. Main-group elements (with some of the lighter transition metals) are the most abundant elements on Earth, in the Solar System, and in the universe. Group 12 elements are often considered to be transition metals; however, zinc (Zn), cadmium (Cd), and mercury (Hg) share some properties of both groups, and some scientists believe they sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
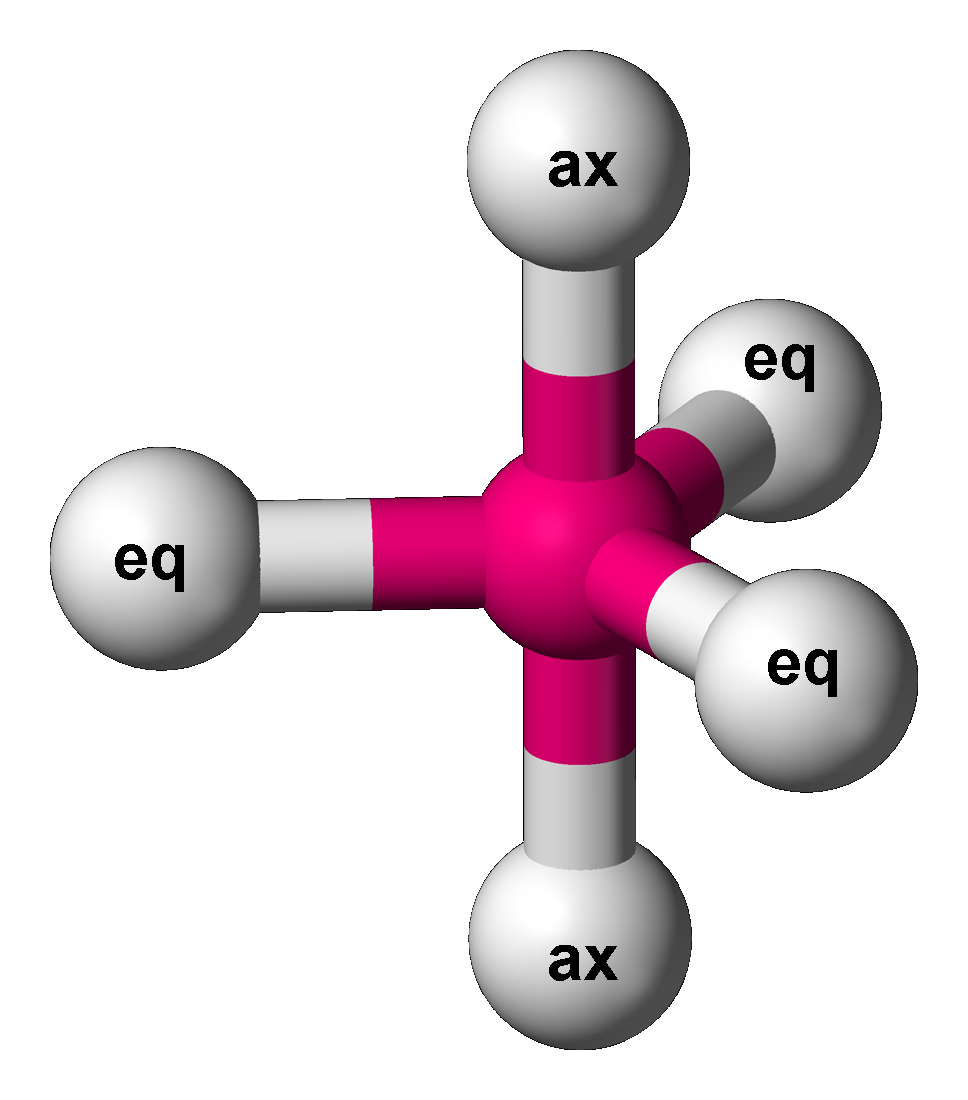
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Lattice
The hexagonal lattice or triangular lattice is one of the five two-dimensional Bravais lattice types. The symmetry category of the lattice is wallpaper group p6m. The primitive translation vectors of the hexagonal lattice form an angle of 120° and are of equal lengths, : , \mathbf a_1, = , \mathbf a_2, = a. The reciprocal lattice of the hexagonal lattice is a hexagonal lattice in reciprocal space with orientation changed by 90° and primitive lattice vectors of length : g=\frac. Honeycomb point set The honeycomb point set is a special case of the hexagonal lattice with a two-atom basis. The centers of the hexagons of a honeycomb form a hexagonal lattice, and the honeycomb point set can be seen as the union of two offset triangular lattices. In nature, carbon atoms of the two-dimensional material graphene are arranged in a honeycomb point set. Crystal classes The ''hexagonal lattice'' class names, Schönflies notation, Hermann-Mauguin notation, orbifold nota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group ( aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009Summary. Ruthenium platinum.matthey.com, p. 9 (2009) to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum |
Group 14
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block. In modern IUPAC notation, it is called group 14. In the field of semiconductor physics, it is still universally called group IV. The group was once also known as the tetrels (from the Greek word ''tetra'', which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). They are also known as the crystallogens or adamantogens. Characteristics Chemical Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior: Each of the elements in this group has 4 electrons in its outer shell. An isolated, neutral group 14 atom has the s2 p2 configuration in the ground state. These elements, especial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Group
The boron group are the chemical elements in group 13 of the periodic table, comprising boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh). The elements in the boron group are characterized by having three valence electrons. These elements have also been referred to as the triels. Boron is commonly classified as a (metalloid) while the rest, with the possible exception of nihonium, are considered post-transition metals. Boron occurs sparsely, probably because bombardment by the subatomic particles produced from natural radioactivity disrupts its nuclei. Aluminium occurs widely on earth, and indeed is the third most abundant element in the Earth's crust (8.3%). Gallium is found in the earth with an abundance of 13 ppm. Indium is the 61st most abundant element in the earth's crust, and thallium is found in moderate amounts throughout the planet. Nihonium is not known to occur in nature and therefore is termed a synthetic element. Several gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 9 Element
Group 9, by modern IUPAC numbering, is a group (column) of chemical elements in the periodic table. Members of Group 9 include cobalt (Co), rhodium (Rh), iridium (Ir) and meitnerium (Mt).Leigh, G. J. ''Nomenclature of Inorganic Chemistry: Recommendations 1990''. Blackwell Science, 1990. . These are all transition metals in the d-block, considered to be some of the most rare of which. Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior; however, rhodium deviates from the pattern. "Group 9" is the modern standard designation for this group, adopted by the IUPAC in 1990. In the older group naming systems, this group was combined with group 8 ( iron, ruthenium, osmium Osmium (from Greek grc, ὀσμή, osme, smell, label=none) is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |