|
Nitric Oxide Dioxygenase
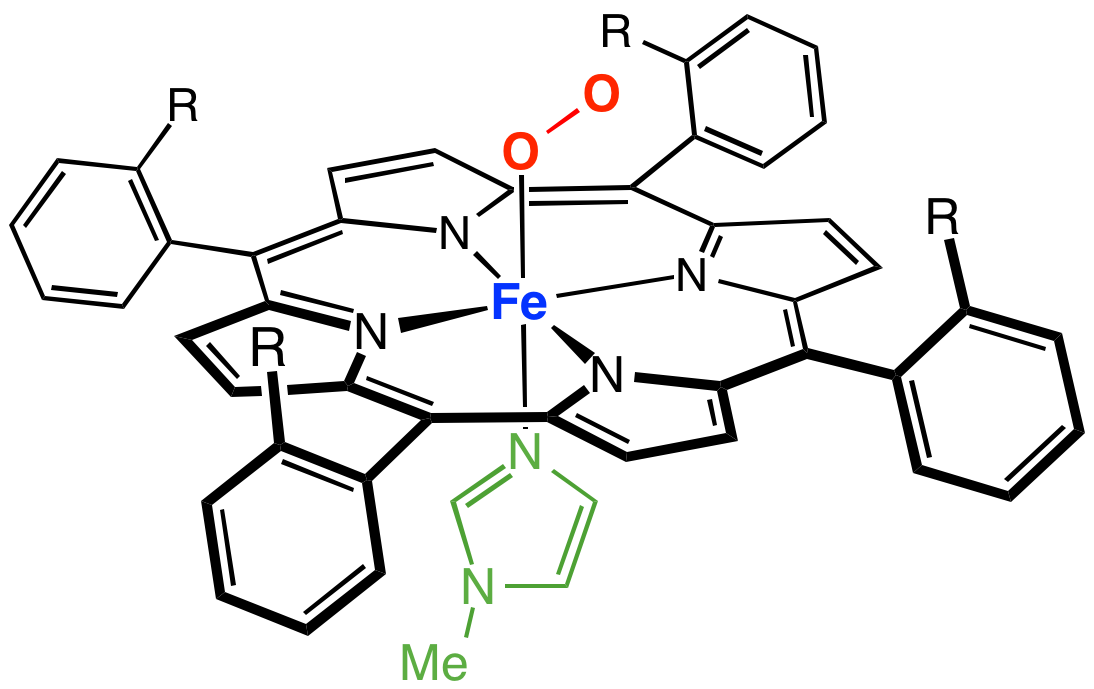
Nitric oxide dioxygenase () is an enzyme that catalyzes the conversion of nitric oxide (NO) to nitrate (NO) . The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below: * 2NO + 2O2 + NAD(P)H → 2NO3− + NAD(P)+ + H+ Nitric oxide is a ubiquitous small molecule that is integrated in a wide variety of physiological processes including smooth muscle vasodilation, platelet disaggregation, neurotransmission, and immune response to bacterial infection. Overproduction of this signaling molecule can be lethal to cells by poisoning cellular energy production. The most sensitive targets of NO are aconitase, an enzyme that catalyzes the isomerization of citrate to isocitrate in the citric acid cycle, and cytochrome oxidase, the last enzyme in the respiratory electron transport chain of mitochondria. Additionally NO, with its lone radical on the nitrogen atom, is implicated in a number of secondary mechanisms of toxicity, including catalase inhibition (resulting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen like hemoglobin does. In humans, myoglobin is only found in the bloodstream after muscle injury. (Google books link is the 2008 edition) High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin. Myoglobin is found in Type I muscle, Type II A, and Type II B; although many texts consider myoglobin not to be found in smooth muscle, this has proved erroneous: there is also myoglobin in smooth muscle cells. Myoglobin was the first protein to have its three-dimensional structure revealed by X-ray crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glioblastoma
Glioblastoma, previously known as glioblastoma multiforme (GBM), is one of the most aggressive types of cancer that begin within the brain. Initially, signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness. The cause of most cases of glioblastoma is not known. Uncommon risk factors include genetic disorders, such as neurofibromatosis and Li–Fraumeni syndrome, and previous radiation therapy. Glioblastomas represent 15% of all brain tumors. They can either start from normal brain cells or develop from an existing low-grade astrocytoma. The diagnosis typically is made by a combination of a CT scan, MRI scan, and tissue biopsy. There is no known method of preventing the cancer. Treatment usually involves surgery, after which chemotherapy and radiation therapy are used. The medication temozolomide is frequently used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers to either one of a pair of isomeric chemical compounds with molecular formula C3H4N2, having a five-membered ring consisting of three carbon atoms and two nitrogen atoms. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodobacter Sphaeroides
''Rhodobacter sphaeroides'' is a kind of purple bacterium; a group of bacteria that can obtain energy through photosynthesis. Its best growth conditions are anaerobic phototrophy (photoheterotrophic and photoautotrophic) and aerobic chemoheterotrophy in the absence of light. ''R. sphaeroides'' is also able to fix nitrogen.De Universiteit van Texas over ''Rhodobacter sphaeroides'' It is remarkably metabolically diverse, as it is able to grow ically via fermentation and |
Felix Hoppe-Seyler
Ernst Felix Immanuel Hoppe-Seyler (''né'' Felix Hoppe; 26 December 1825 – 10 August 1895) was a German physiologist and chemist, and the principal founder of the disciplines of biochemistry and molecular biology. Biography Hoppe-Seyler was born in Freyburg an der Unstrut in the Province of Saxony. He originally trained to be a physician in Halle and Leipzig, and received his medical doctorate from Berlin in 1851. Afterwards, he was an assistant to Rudolf Virchow at the Pathological Institute in Berlin. Hoppe-Seyler preferred scientific research to medicine, and later held positions in anatomy, applied chemistry, and physiological chemistry in Greifswald, Tübingen and Strasbourg. At Strasbourg, he was head of the department of biochemistry, the only such institution in Germany at the time. His work also led to advances in organic chemistry by his students and by immunologist Paul Ehrlich. Among his students and collaborators were Friedrich Miescher (1844–1895) and Nobel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can reach the site of the reaction. * On the other hand, for an enzyme such as cytochrome c peroxidase, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superoxide Dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is ''Lactobacillus plantarum'' and related lactobacilli, which use a different mechanism to prevent damage from reactive . Chemical reaction SODs catalyze the disproportionation of superoxide: : 2 HO2 → O2 + H2O2 In this way, is converted into two less damaging species. The pathway by which SOD-catalyzed dismutation of superoxide may be written, for Cu,Zn SOD, with the following reactions: * Cu2+-SOD + → Cu+-SOD + O2 (reduction of copper; oxidation of superoxide) * Cu+-S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide Evolution 2 Hemoglobin
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate. Dinitrogen complexes The first example of a dinitrogen complex to be discovered was u(NH3)5(N2)sup>2+ (see figure at right), and soon many other such complexes were discovered. These complexes, in which a nitrogen molecule donates at least one lone pair of electrons to a central metal cation, illustrate how N2 might bind to the metal(s) in nitrogenase and the catalyst for the Haber process: these processes involving dinitrogen activation are vitally important in biology and in the production of fertilisers. Dinitrogen is able to coordinate to metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide Evolution
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate. Dinitrogen complexes The first example of a dinitrogen complex to be discovered was u(NH3)5(N2)sup>2+ (see figure at right), and soon many other such complexes were discovered. These complexes, in which a nitrogen molecule donates at least one lone pair of electrons to a central metal cation, illustrate how N2 might bind to the metal(s) in nitrogenase and the catalyst for the Haber process: these processes involving dinitrogen activation are vitally important in biology and in the production of fertilisers. Dinitrogen is able to coordinate to metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |