|
NS5B
Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK (HCV-BK, genotype 1). The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication. Drugs targeting NS5B Several drugs are either on the ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatitis C Virus
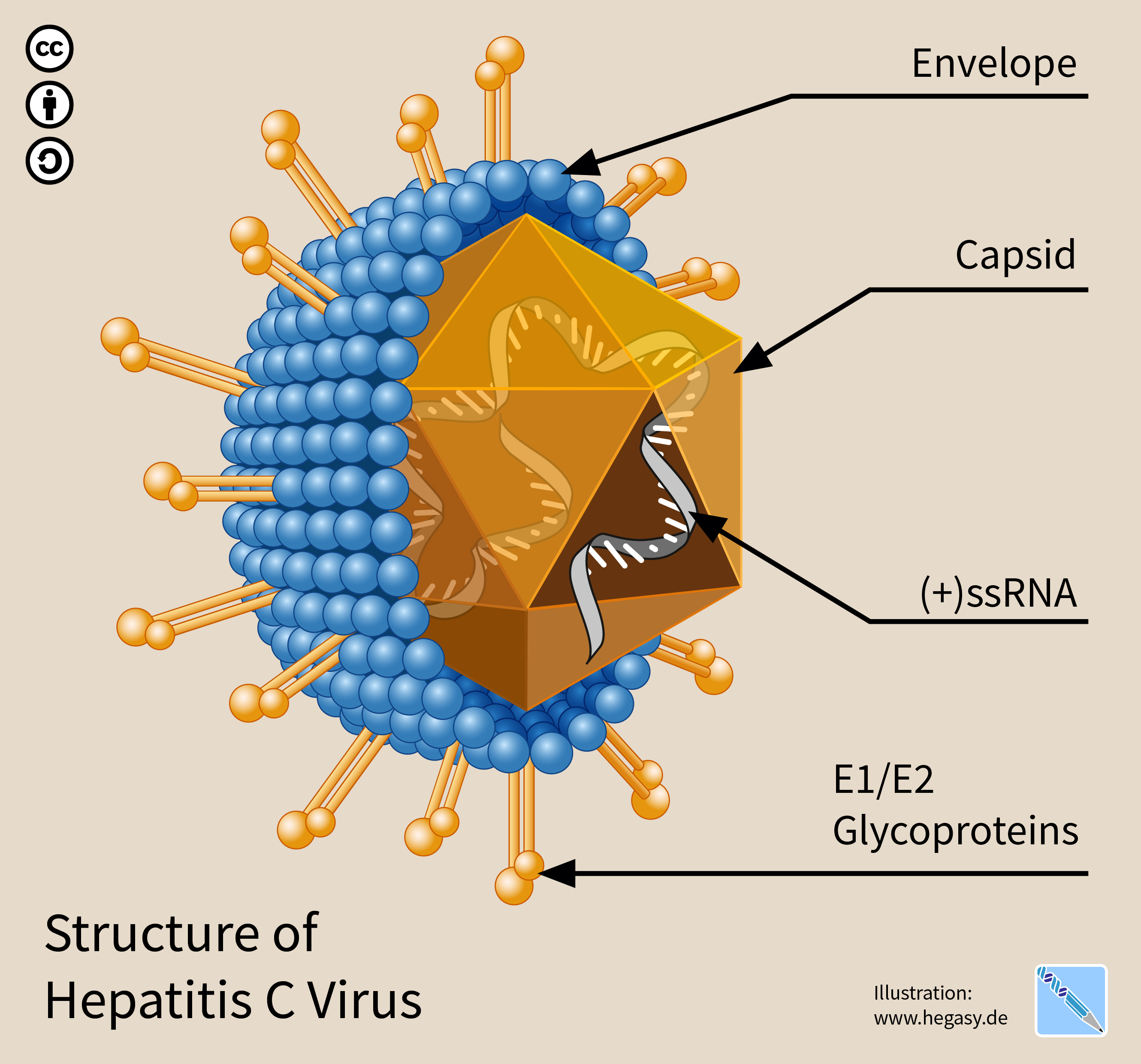
The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family ''Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer ( hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans. Taxonomy The hepatitis C virus belongs to the genus ''Hepacivirus'', a member of the family ''Flaviviridae''. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus. There is also at least one virus in this genus that infects horses. Several additional viruses in the genus have been described in bats and rodents. Structure The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope. They take part in viral attachment and entry into the cell. Within the envel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harvoni
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks. It is generally well tolerated. Common side effects include muscle pains, headache, nausea, rash, and cough. It is unclear if use in pregnancy is safe for the baby. Ledipasvir works by decreasing the activity of NS5A and sofosbuvir works by decreasing the activity of NS5B polymerase. Ledipasvir/sofosbuvir was approved for medical use in the United States, in the European Union, and in Canada in 2014. It is on the World Health Organization's List of Essential Medicines. Medical uses Cure rates are 94% to 99% in people infected with genotype 1 (46% of HCV cases). It has also been evaluated for the treatment of infection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sofosbuvir
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken Oral administration, by mouth. Common side effects include fatigue, headache, nausea, and trouble sleeping. Side effects are generally more common in interferon-containing regimens. Sofosbuvir may reactivate hepatitis B in those who have been previously infected. In combination with ledipasvir, daclatasvir or simeprevir, it is not recommended with amiodarone due to the risk of an bradycardia, abnormally slow heartbeat. Sofosbuvir is in the nucleotide analog family of medications and works by blocking the hepatitis C NS5B protein. Sofosbuvir was discovered in 2007 and approved for medical use in the United States in 2013. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Medical uses Initial HCV treatment In 2016, the American Association for the Study of Liver Diseases and the Infectious Diseases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filibuvir
Filibuvir (also known as PF-00868554, PF-868554) was a non-nucleoside orally available NS5B inhibitor developed by Pfizer for the treatment of hepatitis C. It binds to the non-catalytic Thumb II allosteric pocket of NS5B viral polymerase and causes a decrease in viral RNA synthesis. It is a potent and selective inhibitor, with a mean IC50 The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance ... of 0.019 μM against genotype 1 polymerases. Several filibuvir-resistant mutations have been identified, M423 being the most common that occurred after filibuvir monotherapy. It was intended to be taken twice-daily. Its investigation was discontinued in February 2013 due to strategic reasons. References Abandoned drugs NS5B (polymerase) inhibitors Lactones Triazoles Pyridines Pyrimidin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dasabuvir
Dasabuvir, sold under the brand name Exviera, is an antiviral medication for the treatment of hepatitis C. It is often used together with the combination medication ombitasvir/paritaprevir/ritonavir specifically for hepatitis C virus (HCV) type 1. Ribavirin may also additionally be used. These combinations result in a cure in more than 90% of people. It is taken by mouth. Common side effects include trouble sleeping, nausea, itchiness, and feeling tired. It is not recommended in those with liver failure but appears okay in people with kidney disease. While there is no evidence of harm if used during pregnancy, it has not been well studied. It should not be used with birth control pills that contain ethinylestradiol. Dasabuvir is in the HCV NS5B polymerase inhibitor class of medication. Dasabuvir was approved for medical use in 2014. It is on the World Health Organization's List of Essential Medicines. In the United States, it is approved by the Food and Drug Administration (F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA-dependent RNA Polymerase
RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template. RdRp is an essential protein encoded in the genomes of most RNA-containing viruses with no DNA stage including SARS-CoV-2. Some eukaryotes also contain RdRps, which are involved in RNA interference and differ structurally from viral RdRps. History Viral RdRps were discovered in the early 1960s from studies on mengovirus and polio virus when it was observed that these viruses were not sensitive to actinomycin D, a drug that inhibits cellular DNA-directed RNA synthesis. This lack of sensitivity suggested that there is a virus-specific enzyme that could copy RNA from an RNA template and not from a DNA template. Distr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA Replication
RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template. RdRp is an essential protein encoded in the genomes of most RNA-containing viruses with no DNA stage including SARS-CoV-2. Some eukaryotes also contain RdRps, which are involved in RNA interference and differ structurally from viral RdRps. History Viral RdRps were discovered in the early 1960s from studies on mengovirus and polio virus when it was observed that these viruses were not sensitive to actinomycin D, a drug that inhibits cellular DNA-directed RNA synthesis. This lack of sensitivity suggested that there is a virus-specific enzyme that could copy RNA from an RNA template and not from a DNA template. Distr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NS5A (Hepacivirus)
Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without ''trans''-membrane helices. Structure NS5A is derived from a large polyprotein that is translated from the HCV genome, and undergoes post-translation processing by nonstructural protein 3 (NS3) viral protease. Despite no inherent enzymatic activity being attributed to NS5A, its function is mediated through interaction with other nonstructural (NS) viral and cellular proteins. NS5A has two phosphorylated forms: p56 and p58, which differ in the electrophoretic mobility. p56 is basally phosphorylated by host cellular protein kinase at the center and near the C terminus, whereas p58 is a form of hyper-phosphorylated NS5A at the center of the serine-rich region. Protein mass spectrometry identified several phosphorylated serine residues in this region including serine 225, 229, 232, and 235 res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beclabuvir
Beclabuvir (also known by the research name BMS-791325; abbreviated BCV) is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir (beclabuvir is referred to as BMS-791325 in the trial) on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Setrobuvir
Setrobuvir (also known as ANA-598) was an experimental drug candidate for the treatment of hepatitis C that was discovered at Anadys Pharmaceuticals, which was acquired by Roche in 2011; Roche terminated development in July 2015. It was in Phase IIb clinical trials, used in combination with interferon and ribavirin, targeting hepatitis C patients with genotype 1. Setrobuvir works by inhibiting the hepatitis C enzyme NS5B, an RNA polymerase In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template. Using the enzyme helicase, RNAP locally opens the .... References Abandoned drugs NS5B (polymerase) inhibitors Sulfonamides {{antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radalbuvir
Radalbuvir (INN, also known as GS-9669) is an experimental antiviral drug for the treatment of hepatitis C virus (HCV) infection developed by Gilead Sciences. Radalbuvir acts as an NS5B Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze ... inhibitor. It is currently in clinical trials. It targets NS5B polymerase. References Antiviral drugs Thiophenes Carboxylic acids Experimental drugs Tetrahydrofurans Alkyne derivatives Cyclohexenes Tert-butyl compounds Ethers {{Antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deleobuvir
Deleobuvir (formerly BI 207127) was an experimental drug for the treatment of hepatitis C. It was being developed by Boehringer Ingelheim. It is a non-nucleoside hepatitis C virus NS5B polymerase inhibitor. Deleobuvir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including faldaprevir. Data from the SOUND-C2 study, presented at the 2012 AASLD Liver Meeting, showed that a triple combination of deleobuvir, faldaprevir, and ribavirin performed well in HCV genotype 1b patients. Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients. These results were confirmed in the SOUND-C3 study, presented at the 2013 APASL Liver Conference, which found that 16-week triple therapy with deleobuvir + faldaprevir + ribavirin Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |