|
NLRP12
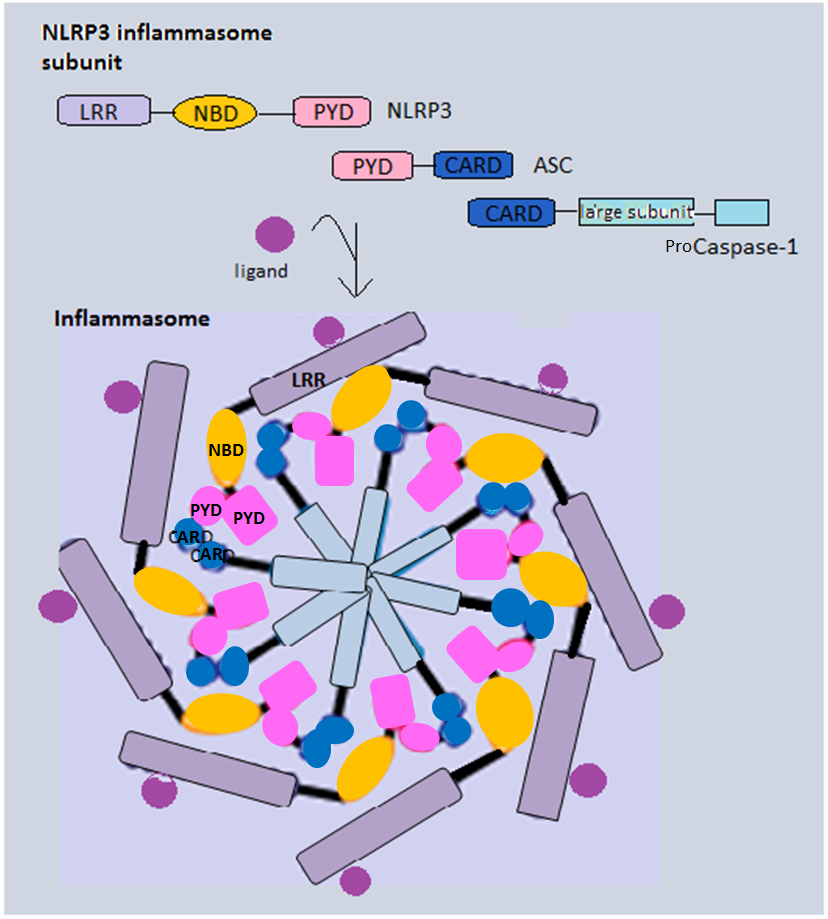
NACHT, LRR and PYD domains-containing protein 12 is a protein that in humans is encoded by the ''NLRP12'' gene. NALPs are cytoplasmic proteins that form a subfamily within the larger CATERPILLER protein family. Most short NALPs, such as NALP12, have an N-terminal pyrin (MEFV; MIM 608107) domain (PYD), followed by a NACHT domain, a NACHT-associated domain (NAD), and a C-terminal leucine-rich repeat ( LRR) region. The long NALP, NALP1 (MIM 606636), also has a C-terminal extension containing a function to find domain (FIIND) and a caspase recruitment domain ( CARD). NALPs are implicated in the activation of proinflammatory caspase Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cyste ...s (e.g., CASP1; MIM 147678) via their involvement in multiprotein complexes called inflammasomes (Tschopp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucleotide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NACHT Domain
The NACHT domain is an evolutionarily conserved protein domain. This NTPase domain is found in apoptosis proteins as well as those involved in MHC transcription. Its name reflects some of the proteins that contain it: NAIP (NLP family apoptosis inhibitor protein), CIITA (that is, C2TA or MHC class II transcription activator), HET-E (incompatibility locus protein from ''Podospora anserina'') and TEP1 (that is, TP1 or telomerase-associated protein). The NACHT domain contains 300 to 400 amino acids. It is a predicted nucleoside-triphosphatase (NTPase) domain, which is found in animal, fungal and bacterial proteins. It is found in association with other domains, such as the CARD domain (), the pyrin domain (), the HEAT repeat domain (), the WD40 repeat (), the leucine-rich repeat (LRR) or the BIR repeat (). The NACHT domain consists of seven distinct conserved motifs, including the ATP/GTPase specific P-loop, the Mg2+-binding site ( Walker A and B motifs, respectively) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol (a gel-like substance), the organelles (the cell's internal sub-structures), and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance or cytoplasmic matrix which remains after exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, the plant plastids, lipid droplets, and vacuoles. Most cellular activities take place within the cytoplasm, such as many metabolic pathways including glycolysis, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEFV
''MEFV'' (Mediterranean fever) is a human gene that provides instructions for making a protein called pyrin (also known as marenostrin). Pyrin is produced in certain white blood cells (neutrophils, eosinophils and monocytes) that play a role in inflammation and in fighting infection. Inside these white blood cells, pyrin is found with the cytoskeleton, the structural framework that helps to define the shape, size, and movement of a cell. Pyrin's protein structure also allows it to interact with other molecules involved in fighting infection and in the inflammatory response. Although pyrin's function is not fully understood, it likely assists in keeping the inflammation process under control. Research indicates that pyrin helps regulate inflammation by interacting with the cytoskeleton. Pyrin may direct the migration of white blood cells to sites of inflammation and stop or slow the inflammatory response when it is no longer needed. The ''MEFV'' gene is located on the short (p) arm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand- turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–protein interactions. Examples Leucine-rich repeat motifs have been identified in a large n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD Domain
Caspase recruitment domains, or caspase activation and recruitment domains (CARDs), are interaction motifs found in a wide array of proteins, typically those involved in processes relating to inflammation and apoptosis. These domains mediate the formation of larger protein complexes via direct interactions between individual CARDs. CARD domains are found on a strikingly wide range of proteins, including helicases, kinases, mitochondrial proteins, caspases, and other cytoplasmic factors. Basic features CARD domains are a subclass of protein motif known as the death fold, which features an arrangement of six to seven antiparallel alpha helices with a hydrophobic core and an outer face composed of charged residues. Other motifs in this class include the pyrin domain (PYD), death domain (DD), and death effector domain (DED), all of which also function primarily in regulation of apoptosis and inflammatory responses. In apoptosis CARD domains were originally characterized based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LRR Proteins
LRR may refer to: *Laminated root rot, a root disease in conifers *Leucine-rich repeat, a type of protein domain * LoadingReadyRun, a Canadian comedy troupe * Long Range Radar * ''Long River Review'', a literary magazine of the University of Connecticut *Low rolling resistance tires, a type of tires designed for fuel efficiency * Light Reaction Regiment, the Philippine Army counter-terrorist unit modeled after the U.S. Army Delta Force and British SAS * Loose Round Robin, Warp Scheduling See also * LR (other) LR or Lr may refer to: Businesses and organizations *Avianca Costa Rica, an airline, IATA airline code LR *Lenoir–Rhyne University in Hickory, North Carolina * Lenong Regiment, an infantry regiment of the South African Army *The Republicans (Fr ... * Lrrr (other) {{dab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |