|
NLRP11
NOD-like receptor family pyrin domain containing 11 is a protein that in humans is encoded by the NLRP11 gene located on the long arm of human chromosome 19q13.42. NLRP11 belongs to the NALP subfamily, part of a large subfamily of caterpiller. It is also known as NALP11, PYPAF6, NOD17, PAN10, and CLR19.6 Being a member of the NOD-like receptor protein (NLRP) gene family, it encodes a protein with an N-terminal pyrin death (PYD) domain and nucleoside triphosphate hydrolase ( NACHT) domain and a C-terminal leucine-rich repeats (LRR) region. This gene regulates caspases in the proinflammatory signal transduction pathway. Based on studies of other members of the NLRP gene family with similar domain structures, it is predicted to form part of the multiprotein inflammasome complex. NLRP11 is expressed mainly in immune cells, B cells, myeloid cells, and B cell lymphoma cell lines. NLRP11 is involved in the regulation of inflammatory responses in human cells. NALPs family controls cyto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NOD-like Receptor
The nucleotide-binding oligomerization domain-like receptors, or NOD-like receptors (NLRs) (also known as nucleotide-binding leucine-rich repeat receptors), are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores, and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs), and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response. They are found in lymphocytes, macrophages, dendritic cells and also in non-immune cells, for example in epithelium. NLRs are highly conserved through evolution. Their homologs have been discovered in many different animal species (APAF1) and also in the plant kingdom ( disease-resistance R protein). Structure NLRs contain 3 domains – central NACHT (NOD or NBD – nucleotide-binding domain) domai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NACHT Domain
The NACHT domain is an evolutionarily conserved protein domain. This NTPase domain is found in apoptosis proteins as well as those involved in MHC transcription. Its name reflects some of the proteins that contain it: NAIP (NLP family apoptosis inhibitor protein), CIITA (that is, C2TA or MHC class II transcription activator), HET-E (incompatibility locus protein from ''Podospora anserina'') and TEP1 (that is, TP1 or telomerase-associated protein). The NACHT domain contains 300 to 400 amino acids. It is a predicted nucleoside-triphosphatase (NTPase) domain, which is found in animal, fungal and bacterial proteins. It is found in association with other domains, such as the CARD domain (), the pyrin domain (), the HEAT repeat domain (), the WD40 repeat (), the leucine-rich repeat (LRR) or the BIR repeat (). The NACHT domain consists of seven distinct conserved motifs, including the ATP/GTPase specific P-loop, the Mg2+-binding site ( Walker A and B motifs, respectively) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromosome
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. In most chromosomes the very long thin DNA fibers are coated with packaging proteins; in eukaryotic cells the most important of these proteins are the histones. These proteins, aided by chaperone proteins, bind to and condense the DNA molecule to maintain its integrity. These chromosomes display a complex three-dimensional structure, which plays a significant role in transcriptional regulation. Chromosomes are normally visible under a light microscope only during the metaphase of cell division (where all chromosomes are aligned in the center of the cell in their condensed form). Before this happens, each chromosome is duplicated ( S phase), and both copies are joined by a centromere, resulting either in an X-shaped structure (pictured above), if the centromere is located equatorially, or a two-arm structure, if the centromere is located distally. The joined copies are now called si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caterpiller Family
Caterpillars ( ) are the larval stage of members of the order Lepidoptera (the insect order comprising butterflies and moths). As with most common names, the application of the word is arbitrary, since the larvae of sawflies (suborder Symphyta) are commonly called caterpillars as well. Both lepidopteran and symphytan larvae have eruciform body shapes. Caterpillars of most species eat plant material ( often leaves), but not all; some (about 1%) eat insects, and some are even cannibalistic. Some feed on other animal products. For example, clothes moths feed on wool, and horn moths feed on the hooves and horns of dead ungulates. Caterpillars are typically voracious feeders and many of them are among the most serious of agricultural pests. In fact, many moth species are best known in their caterpillar stages because of the damage they cause to fruits and other agricultural produce, whereas the moths are obscure and do no direct harm. Conversely, various species of cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 11
Murine caspase-11, and its human homologs caspase-4 and caspase-5, are mammalian intracellular receptor proteases activated by TLR4 and TLR3 signaling during the innate immune response. Caspase-11, also termed the non-canonical inflammasome, is activated by TLR3/ TLR4-TRIF signaling and directly binds cytosolic lipopolysaccharide (LPS), a major structural element of Gram-negative bacterial cell walls. Activation of caspase-11 by LPS is known to cause the activation of other caspase proteins, leading to septic shock, pyroptosis, and often organismal death. History LPS is a known activator of innate immune responses. Extracellular LPS binds specifically to the cell surface receptor TLR4. LPS binding to TLR4 subsequently causes initiation of the MyD88 and TRIF signaling pathways, leading to expression of pro- inflammatory molecules and cytokines. These inflammatory mediators cause host toxic shock and sepsis as a result of an overactive immune response to LPS. Until re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 4
Caspase 4 is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue (LEVD-), and belongs to a family of cysteine proteases called caspases. The function of caspase 4 is not fully known, but it is believed to be an inflammatory caspase, along with caspase 1, caspase 5 (and the murine homolog caspase 11), with a role in the immune system. The anti-inflammatory drug indoprofen is an inhibitor of the activity of the caspase-4 enzyme. See also * The Proteolysis Map * Caspase References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for peptidases and their inhibitorsC14.007 EC 3.4.22 Caspases {{gene-11-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrial Antiviral-signaling Protein
Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the outer membrane of the mitochondria, peroxisomes, and mitochondrial-associated endoplasmic reticulum membrane (MAM). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus. Structure MAVS is also known as IFN-β promoter stimulator I (IPS-1), caspase activation recruitment domain adaptor inducing I FN-β(CARDIF), or virus induced signaling adaptor (VISA). MAVS is encoded by a ''MAVS'' gene. MAVS is a 540 amino acid protein that consists of three components, a N terminal caspase activation recruitment domain (CARD), a proline rich domain, and a transmembrane C terminal domain (TM ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DDX3X
ATP-dependent RNA helicase DDX3X is an enzyme that in humans is encoded by the ''DDX3X'' gene. Function DEAD box proteins, characterized by the conserved motif Asp-Glu-Ala-Asp (DEAD), are putative RNA helicases. They are implicated in a number of cellular processes involving alteration of RNA secondary structure such as translation initiation, nuclear and mitochondrial splicing, and ribosome and spliceosome assembly. Based on their distribution patterns, some members of this family are believed to be involved in embryogenesis, spermatogenesis, and cellular growth and division. This gene encodes a DEAD box protein, which interacts specifically with hepatitis C virus core protein resulting a change in intracellular location. This gene has a homolog located in the nonrecombining region of the Y chromosome. The protein sequence is 91% identical between this gene and the Y-linked homolog. Sub-cellular trafficking DDX3X performs its functions in the cell nucleus and cytoplasm, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
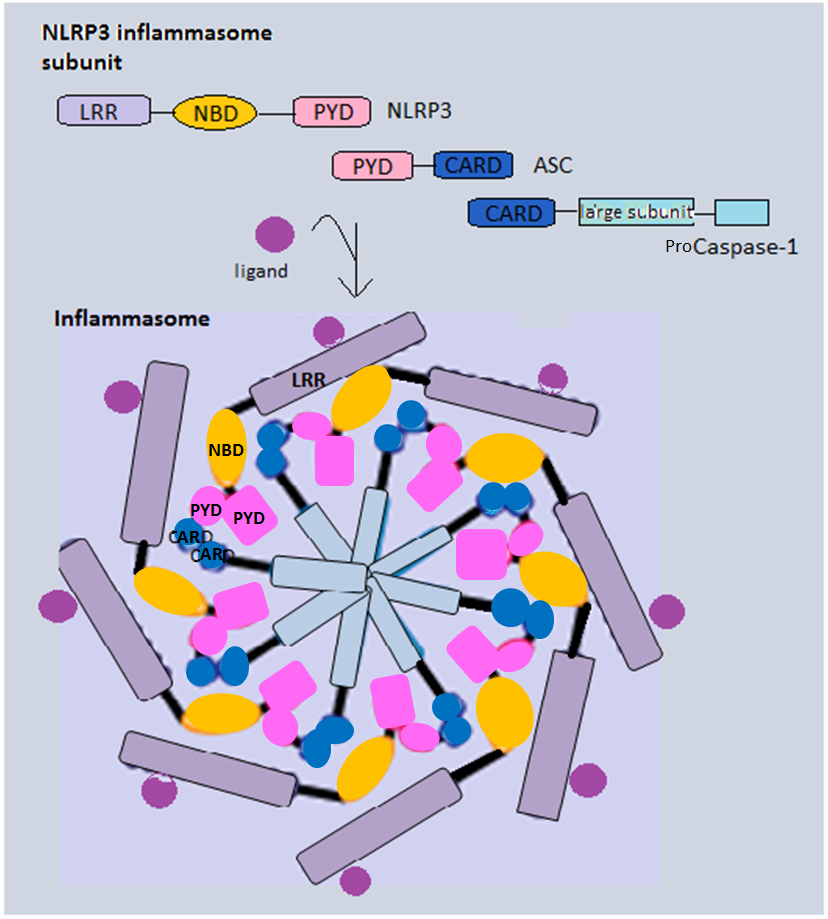
NLRP3 Inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucleotide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |