|
Non-degenerate Two-photon Absorption
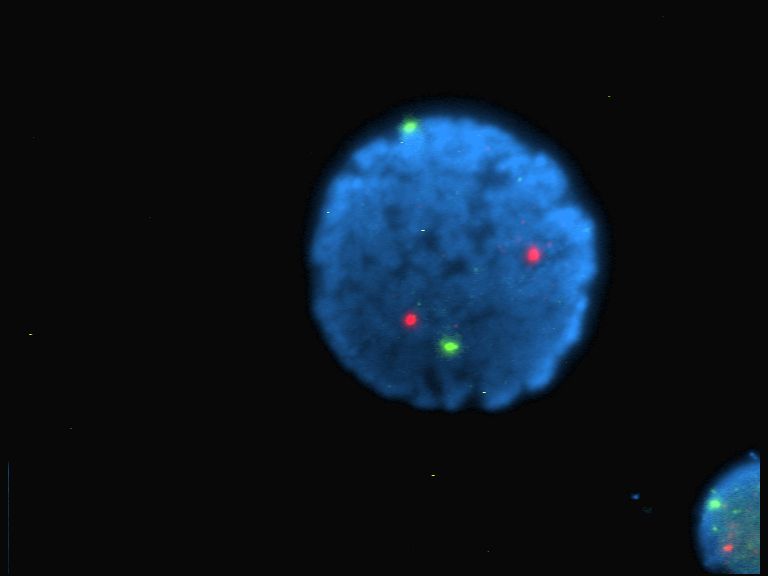
Non-degenerate two-photon absorption (ND-TPA or ND-2PA) or two-color two-photon excitation is a type of two-photon absorption (TPA) where two photons with different energies are (almost) simultaneously absorbed by a molecule, promoting a molecular electronic transition from a lower energy state A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The te ... to a higher energy state. The sum of the energies of the two photons is equal to, or larger than, the total energy of the transition. The probability of ND-TPA is quantified as the non-degenerate two-photon absorption cross section (ND-TPACS) and is an inherent property of molecules. ND-TPACS has been measured using Z-scan ( pump-probe) techniques, which measure the laser intensity decrease due to absorption, and fluorescence-based techn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Two Photon Excitation Diagram
2 (two) is a number, numeral and digit. It is the natural number following 1 and preceding 3. It is the smallest and only even prime number. Because it forms the basis of a duality, it has religious and spiritual significance in many cultures. Evolution Arabic digit The digit used in the modern Western world to represent the number 2 traces its roots back to the Indic Brahmic script, where "2" was written as two horizontal lines. The modern Chinese and Japanese languages (and Korean Hanja) still use this method. The Gupta script rotated the two lines 45 degrees, making them diagonal. The top line was sometimes also shortened and had its bottom end curve towards the center of the bottom line. In the Nagari script, the top line was written more like a curve connecting to the bottom line. In the Arabic Ghubar writing, the bottom line was completely vertical, and the digit looked like a dotless closing question mark. Restoring the bottom line to its original horizontal posi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Two-photon Absorption
Two-photon absorption (TPA or 2PA) or two-photon excitation or non-linear absorption is the simultaneous absorption of two photons of identical or different frequencies in order to excite a molecule from one state (usually the ground state) to a higher energy, most commonly an excited electronic state. Absorption of two photons with different frequencies is called non-degenerate two-photon absorption. Since TPA depends on the simultaneous absorption of two photons, the probability of TPA is proportional to the square of the light intensity, thus it is a nonlinear optical process. The energy difference between the involved lower and upper states of the molecule is equal or smaller than the sum of the photon energies of the two photons absorbed. Two-photon absorption is a third-order process, with absorption cross section typically several orders of magnitude smaller than one-photon absorption cross section. Two-photon excitation of a fluorophore (a fluorescent molecule) leads to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are Massless particle, massless, so they always move at the speed of light, speed of light in vacuum, (or about ). The photon belongs to the class of bosons. As with other elementary particles, photons are best explained by quantum mechanics and exhibit wave–particle duality, their behavior featuring properties of both waves and particles. The modern photon concept originated during the first two decades of the 20th century with the work of Albert Einstein, who built upon the research of Max Planck. While trying to explain how matter and electromagnetic radiation could be in thermal equilibrium with one another, Planck proposed that the energy stored within a material object should be regarded as composed of an integer number of discrete, equal-sized parts. To explai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon Energy
Photon energy is the energy carried by a single photon. The amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. The higher the photon's frequency, the higher its energy. Equivalently, the longer the photon's wavelength, the lower its energy. Photon energy can be expressed using any unit of energy. Among the units commonly used to denote photon energy are the electronvolt (eV) and the joule (as well as its multiples, such as the microjoule). As one joule equals 6.24 × 1018 eV, the larger units may be more useful in denoting the energy of photons with higher frequency and higher energy, such as gamma rays, as opposed to lower energy photons as in the optical and radio frequency regions of the electromagnetic spectrum. Formulas Physics Photon energy is directly proportional to frequency. E = hf where *E is energy *h is the Planck constant * f is frequency This equation is know ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Electronic Transition
Molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy level. The energy change associated with this transition provides information on the structure of a molecule and determines many molecular properties such as colour. The relationship between the energy involved in the electronic transition and the frequency of radiation is given by Planck's relation. Organic molecules and other molecules The electronic transitions in organic compounds and some other compounds can be determined by ultraviolet–visible spectroscopy, provided that transitions in the ultraviolet (UV) or visible range of the electromagnetic spectrum exist for this compound. Electrons occupying a HOMO of a sigma bond can get excited to the LUMO of that bond. This process is denoted as a σ → σ* transition. Likewise promotion of an electron from a π-bonding orbital to an antibonding π orbital* is denoted as a π → π* transition. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Cross Section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc. (Note that light in this context is described as consisting of particles, i.e., photons.) In honor of the fundamental contribution of Maria Goeppert Mayer to this area, the unit for the two-photon absorption cross section is named the "GM". One GM is 10−50 cm4⋅s⋅photon−1. In the context of ozone shielding of ultraviolet light, absorption cross section is the ability of a molecule to absorb a photon of a particular wavelength and polarization. Analogously, in the context of nuclear engineering it refers to the probability of a particle (usually a neutron) being absorbed by a nucleus. Although the units are given as an area, it does not refer to an actual size area, at least partially because the density o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents ( antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uncertainty Principle
In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as position, ''x'', and momentum, ''p'', can be predicted from initial conditions. Such variable pairs are known as complementary variables or canonically conjugate variables; and, depending on interpretation, the uncertainty principle limits to what extent such conjugate properties maintain their approximate meaning, as the mathematical framework of quantum physics does not support the notion of simultaneously well-defined conjugate properties expressed by a single value. The uncertainty principle implies that it is in general not possible to predict the value of a quantity with arbitrary certainty, even if all initial conditions are specified. Introduced first in 1927 by the German physicist Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
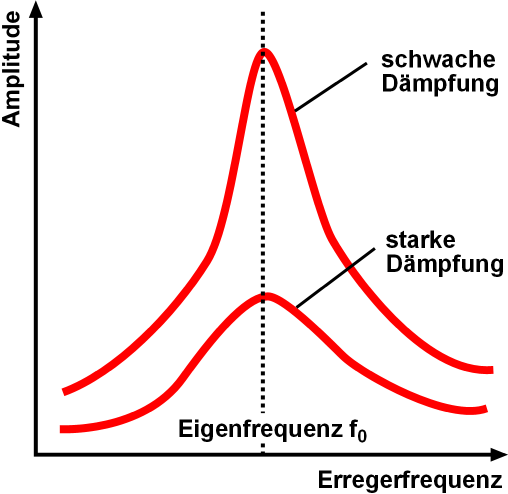
Resonance Enhancement
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |