|
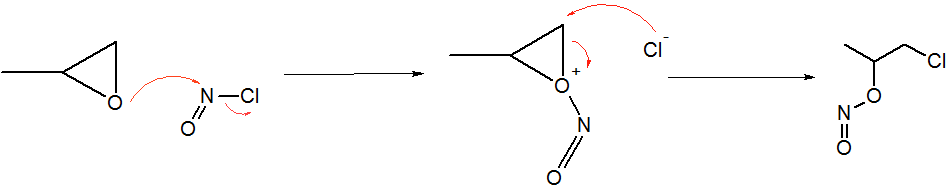
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroxyl
Nitroxyl (common name) or azanone (IUPAC name) is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in the solution phase. The conjugate base, NO−, nitroxide anion, is the redox reaction, reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. The bond dissociation energy of H−NO is , which is unusually weak for a bond to the hydrogen atom. Generation Nitroxyl is produced from the reagents Angeli's salt (Na2N2O3) and Piloty's acid (PhSO2NHOH). Other notable studies on the production of HNO exploit cycloadducts of acyl nitroso species, which are known to decompose via hydrolysis to HNO and acyl acid. Upon photodissociation, photolysis these compounds release the acyl nitroso species which then further decompose. HNO is generated via organic oxidation of oxime, cyclohexanone oxime with lead tetraacetate to form 1-nitrosocyclohexyl acetate: : This compound can be hydrolyzed under base (chemistry), bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ... (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern molecular orbital theory, theories of chemical bonding. An important Reaction intermediate, intermediate in chemical industry, industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum Hexacarbonyl
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state. Structure and properties Mo(CO)6 adopts an octahedral geometry consisting of six rod-like CO ligands radiating from the central Mo atom. A recurring minor debate in some chemical circles concerns the definition of an "organometallic" compound. Usually, organometallic indicates the presence of a metal directly bonded via a M–C bond to an organic fragment, which must in turn have a C–H bond. Mo(CO)6 is prepared by the reduction of molybdenum chlorides or oxides under a pressure of carbon monoxide, although it would be unusual to prepare this inexpensive compound in the laboratory. The compound is somewhat air-stable and sparingly soluble in nonpolar organic solvents. Occurrence Mo(CO)6 has been detected in landfil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Nitrosyl Complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat hypertension. Metal nitrosyl complexes are complex (chemistry), complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrated by the isoelectronic pair Fe(CO)2(NO)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Cyanide
Nitrosyl cyanide, a blue-green gas, is the compound with the molecular formula ONCN. The compound has been invoked as a product of the oxidation of cyanamide catalyzed by the enzyme glucose oxidase. Structure, synthesis, reactivity The structure of nitrosyl cyanide is planar. It is strongly bent at the internal nitrogen, analogous to the structure of nitrosyl chloride. The C-N-O angle is 113°. The NCN angle is 170°. The compound can be created by the reaction of nitrosyl chloride and silver cyanide at low temperatures. It is not typically isolated, but trapped by Diels-Alder reactions, e.g. with butadiene. Cycloadditions occur across the N=O bond. It forms a reversible adduct with 9,10-dimethylantracene. Related compound * Nitryl cyanide (O2NCN), a colorless gas (b.p. 7 °C).{{cite journal , doi=10.1002/anie.201404209, title=Nitryl Cyanide, NCNO2, year=2014, last1=Rahm, first1=Martin, last2=Bélanger-Chabot, first2=Guillaume, last3=Haiges, first3=Ralf, last4=Christe, first4= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms ''Ps''–''Ps'' or ''Ps''–X (where ''Ps'' is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferouscyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide. Common pseudohalogens and their nomenclature Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison): Au− is considered to be a pseudohalogen ion due to its disproportionation reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration to some samples. AgCl occurs naturally as a mineral chlorargyrite. Preparation Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis: combining an aqueous solution of silver nitrate (which is soluble) with a soluble chloride salt, such as sodium chloride or cobalt(II) chloride. The silver chloride that forms will precipitate immediately. :AgNO3 + NaCl -> AgCl(v) + NaNO3 :2 AgNO3 + CoCl2 -> 2 AgCl(v) + Co(NO3)2 Structure and reactions The solid adopts the ''fcc'' NaCl structure, in which each Ag+ ion is surrounded by an octahedron of six chloride liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid. In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction. In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. Nomenclature The nomenclature of organic acid anhydrides is derived from the names of the constituent carboxylic acids which underwent dehydration to form the compound. In symmetrical acid anhydrides, where only one constituent carboxylic acid was used to form the compound (such as the dehydration of propanoic acid, 2CH3CH2COOH → CH3CH2C(O)OC(O)CH2CH3 + H2O), only the prefix of the original carboxylic acid is used and the suffix "anhydride" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosonium
The nitrosonium ion is , in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: , (nitrosylsulfuric acid, more descriptively written ) and . The and salts are slightly soluble in acetonitrile . NOBF4 can be purified by sublimation at 200–250 °C and . is isoelectronic with CO, and . It arises via protonation of nitrous acid: :HONO + H+ NO+ + H2O Chemical properties Hydrolysis reacts readily with water to form nitrous acid: : For this reason, nitrosonium compounds must be protected from water or even moist air. With base, the reaction generates nitrite: : As a diazotizing agent reacts with aryl amines, , to give diazonium salts, . The resulting diazonium group is easily displaced (unlike the amino group) by a variety of nucleophiles. As an oxidizing agent , e.g. as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers. Preparation and structure Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride: :SbCl3 + Cl2 → SbCl5 Gaseous SbCl5 has a trigonal bipyramidal structure. Reactions This compounds reacts with water to form antimony pentoxide and hydrochloric acid: :2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O. This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity. It is also a strong oxidizing agent. For example aromatic ethers are oxidized to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |