|
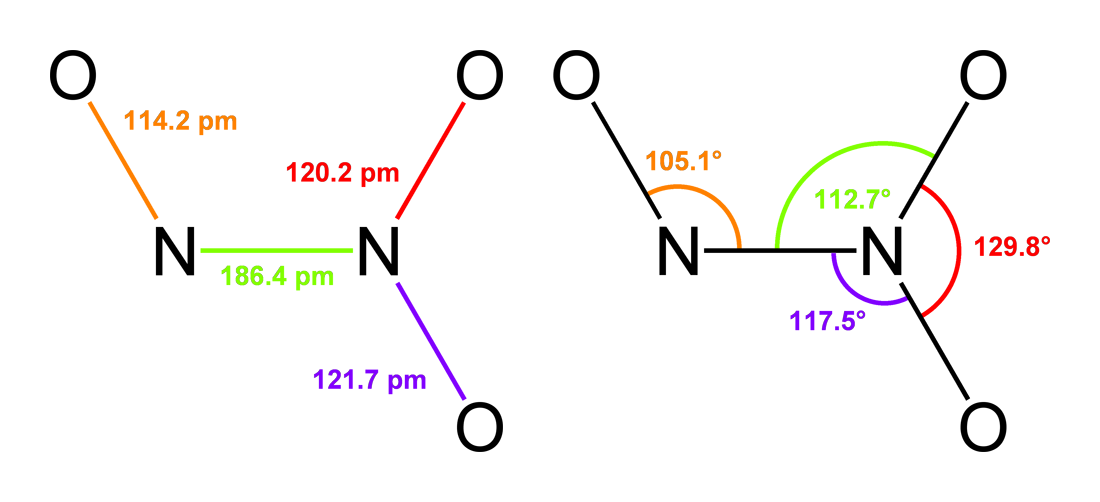
Nitrogen(III) Oxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. It is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F): :NO + NO2 N2O3 Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. In liquid and solid states, it has a deep blue color. At higher temperatures the equilibrium favors the constituent gases, with ''K''diss = 193 kPa (25 °C). This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical . Structure and bonding Typically, N–N bonds are similar in length to that in hydrazine (145 pm). Dinitrogen trioxide, however, has an unusually long N–N bond at 186 pm. Some other nitrogen oxides also possess long N–N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmetry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry. It is included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidic Oxides
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the generation of carbonic acid) when dissolved. The acidity of an oxide can be reasonably assumed by its accompanying constituents. Less electronegative elements tend to form basic oxides such as sodium oxide and magnesium oxide, whereas more electronegative elements tend to produce acidic oxides as seen with carbon dioxide and phosphorus pentoxide. Some oxides like aluminium oxides are amphoteric. Acidic oxides are of environmental concern. Sulfur and nitrogen oxides are considered air pollutants as they react with atmospheric water vapour to produce acid rain. Examples Carbonic acid is an illustrative example of the Lewis acidity of an acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxides
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide *Nitrogen dioxide (), nitrogen(IV) oxide *Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide *Dinitrogen dioxide (), nitrogen(II) oxide Dimer (chemistry), dimer *Dinitrogen trioxide (), nitrogen(II,IV) oxide *Dinitrogen tetroxide (), nitrogen(IV) oxide Dimer (chemistry), dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate *Nitrosyl azide (), nitrogen(−I,0,I,II) oxide *Nitryl azide () *Oxatetrazole () *Trinitramide ( or ), nitrogen(0,IV) oxide Anions *Nitroxyl, Nitroxide () *Nitrite ( or ) *Nitrate () *Peroxynitrite ( or ) *Peroxynitrate ( or ) *Orthonitrate (, analogous to phosphate ) *Hyponitrite ( or ) *Trioxodinitrate or hyponitrate ( or ) *Nitroxylate ( or ) *Ammonium dinitramide, Dinitramide ( or ) Cations *Nitrosonium ( or ) *Nitronium ( or ) Atm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in Solution (chemistry), solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes. Structure In the gas phase, the planar nitrous acid molecule can adopt both a ''syn'' and an ''anti'' form. The ''anti'' form predominates at room temperature, and infrared spectroscopy, IR measurements indicate it is Gibbs free energy, more stable by around 2.3 kJ/mol. p. 462. Image:Trans-nitrous-acid-2D-dimensions.png , Dimensions of the ''anti'' form(from the rotational spectroscopy, microwave spectrum) Image:Trans-nitrous-acid-3D-balls.png , ball-and-stick model, Model of the ''anti'' form Image:Cis-nitrous-acid-3D-balls.png , ''syn'' form Preparation Nitrous acid is usually generated by acidification of aqueous solutions of sodium nitrite with a mineral acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word ''acid'' in the name of the parent carboxylic acid by the word ''anhydride''. Thus, (CH3CO)2O is called ''acetic anhydride.'' Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride"). One or both acyl groups of an acid anhydride may also be d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets. Structure and properties Dinitrogen tetroxide could be regarded as two nitro groups (-NO2) bonded together. It forms an equilibrium mixture with nitrogen dioxide. The molecule is planar with an N-N bond distance of 1.78Å and N-O distances of 1.19Å. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.45Å. This exceptionally weak σ bond (amounting to overlapping of the ''sp''2 hybrid orbitals of the two NO2 units) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Trioxide
Trioxidonitrogen(•) or nitrate radical is an oxide of nitrogen with formula , consisting of three oxygen atoms covalent bond, covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems. Like nitrogen dioxide , it is a radical (chemistry), radical (a molecule with an unpaired valence electron), which makes it paramagnetism, paramagnetic. It is the uncharged counterpart of the nitrate anion and an isomer of the peroxynitrite radical . Nitrogen trioxide is an important intermediate in reactions between atmospheric components, including the destruction of ozone.R. P. Wayne, I. Barnes, P. Biggs, J. P. Burrows, C. E. Canosa-Mas, J. Hjorth, G. Le Bras. G. K. Moortgat, D. Perner, G. Poulet, G. Restelli, and H. Sidebottom (1991): "The nitrate radical: Physics, chemistry, and the atmosphere". ''Atmospheric Environment. Part A. General Topics''. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently encount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |