|
Niobium(V) Ethoxide
Niobium(V) ethoxide is an metalorganic compound with formula Nb2(OC2H5)10. It is a colorless liquid that dissolves in some organic solvents but hydrolyzes readily.W. M. Haynes. CRC Handbook of Chemistry and Physics, 93rd Edition. Physical Constants of Inorganic Compounds. It is mainly used for the sol-gel processing of materials containing niobium oxides. Structure Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception. Early studies established that niobium alkoxides aggregate in solution as dimers. Subsequent crystallographic analysis established that the methoxide and isopropoxides of niobium adopt bioctahedral structures. From a geometric perspective, the ten ethoxide ligand oxygen atoms of the Nb2(OEt)10 molecule in solution define a pair of octahedra sharing a common edge with the two niobium atoms located at their centres. From a bonding perspective, each niobium centre is surrounded octahedrally by four monodentate and two bridging ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt Metathesis
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme: :AB + CD -> AD + CB The bond between the reacting species can be either ionic or covalent. Classically, these reactions result in the precipitation of one product. In older literature, the term double decomposition is frequently encountered. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (substrate) is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: monocrystalline, polycrystalline, amorphous, and epitaxial. These materials include: silicon ( dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond and graphene), fluorocarbons, filaments, tungsten, titanium nitride and various high-κ dielectrics. The term ''chemical vapour deposition'' was coined 1960 by ''John M. Blocher, Jr.'' who intended to differentiate ''chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Layer Deposition
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas-phase chemical process; it is a subclass of chemical vapour deposition. The majority of ALD reactions use two chemicals called precursors (also called "reactants"). These precursors react with the surface of a material one at a time in a sequential, self-limiting, manner. A thin film is slowly deposited through repeated exposure to separate precursors. ALD is a key process in fabricating semiconductor devices, and part of the set of tools for synthesising nanomaterials. Introduction During atomic layer deposition a film is grown on a substrate by exposing its surface to alternate gaseous species (typically referred to as precursors or reactants). In contrast to chemical vapor deposition, the precursors are never present simultaneously in the reactor, but they are inserted as a series of sequential, non-overlapping pulses. In each of these pulses the precursor molecules react wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium(V) Oxide
Niobium pentoxide is the inorganic compound with the formula Nb2 O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.Francois Cardarelli (2008) ''Materials Handbook'' Springer London Structure It has many polymorphic forms all based largely on octahedrally coordinated niobium atoms.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications The polymorphs are identified with a variety of prefixes. The form most commonly encountered is monoclinic H- Nb2 O5, which has a complex structure with a unit cell containing 28 niobium atoms and 70 oxygen, where 27 of the niobium atoms are octahedrally coordinated and one tetrahedrally. There is an uncharacterised solid hydrate, , the so-called niobic acid (previously cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile (ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium Pentachloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation. Structure and properties Niobium(V) chloride forms chloro-bridged dimers in the solid state (''see'' figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, TaCl5 and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures. Preparation Industrial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ways to the first coordination sphere. First coordination sphere The first coordination sphere refers to the molecules that are attached directly to the metal. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes, ion pairing is important. In hexamminecobalt(III) chloride ( o(NH3)6l3), the cobalt cation plus the 6 ammonia ligands comprise the first coordination sphere. The coordination sphere of this ion thus consists of a central MN6 core "decorated" by 18 N−H bonds that radiate outwards. Second coordination sphere Metal ions can be described as consisting of series of two concentric coordination spheres, the first and second. More distant from the second coo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
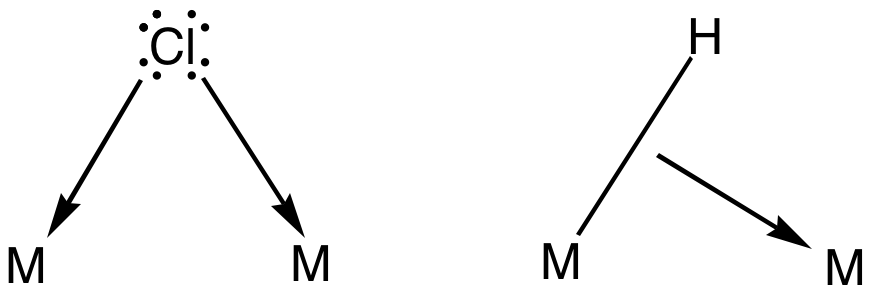
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |