|
Newman–Kwart Rearrangement
The Newman–Kwart rearrangement is a type of rearrangement reaction in which the aryl group of an ''O''-aryl thiocarbamate, ArOC(=S)NMe2, migrates from the oxygen atom to the sulfur atom, forming an ''S''-aryl thiocarbamate, ArSC(=O)NMe2. The reaction is named after its discoverers, Melvin Spencer Newman and Harold Kwart. The reaction is a manifestation of the double bond rule. : Mechanism The Newman–Kwart rearrangement is intramolecular; it proceeds ''via'' a four-membered cyclic transition state. : Use for preparation of thiophenols The Newman–Kwart rearrangement is an important prelude to the synthesis of thiophenols. A phenol (1) is deprotonated with a base followed by treatment with a thiocarbamoyl chloride (2) to form an ''O''-aryl thiocarbamate (3). Heating 3 to around 250 °C causes it undergo Newman–Kwart rearrangement to an ''S''-aryl thiocarbamate (4). Alkaline hydrolysis or similar cleavage yields a thiophenol (5). : See also * Smiles rearrangement * Cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melvin Spencer Newman
Melvin Spencer Newman (March 10, 1908 – May 30, 1993) was an American chemist, Ohio State University professor, best known for inventing the Newman projection. Newman was born in New York City in a Jewish family, the youngest of Mae (née Polack) and Jacob K. Newman's four children."Newman, Jacob K.," in: James Terry White, ''The National Cyclopaedia of American Biography'', v. 33, New York: J. T. White, 1947, p. 183. His paternal grandfather was the New Orleans German-born investment banker and philanthropist Isidore Newman. Shortly after his birth, his family moved to New Orleans, Louisiana. When he was 14, they moved back to New York, where he attended Riverdale County School. From 1925 to 1932, he attended Yale University, where he obtained his B.A. ''magna cum laude'' in 1929 and his Ph.D in 1932, under the direction of Professor Rudolph J. Anderson. He was a member of Zeta Beta Tau. After postdoctoral stays at Yale, Columbia University and Harvard University, he bega ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Reaction
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place. Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
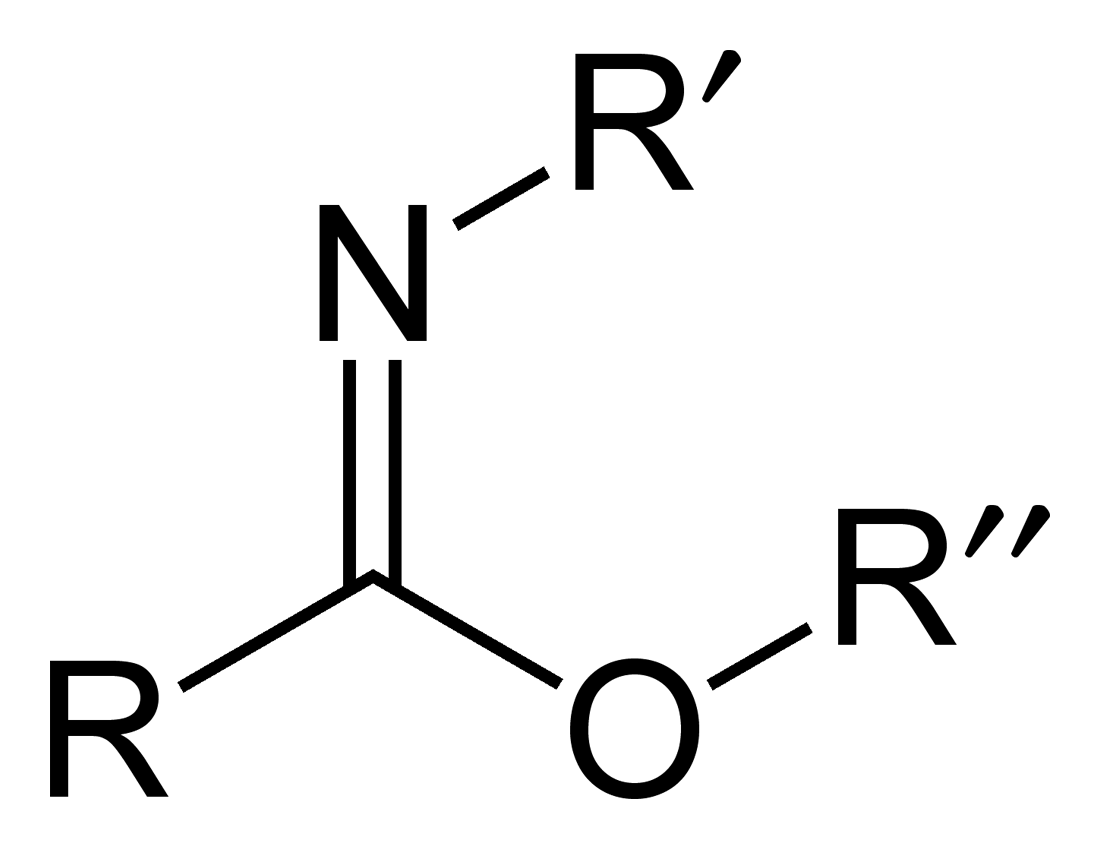
Chapman Rearrangement
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between a carboximidic acid (R-C(=NR')OH) and an alcohol, with the general formula R-C(=NR')OR". They are also known as imino ethers, since they resemble imines (>C=N-) with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smiles Rearrangement
The Smiles rearrangement is an organic reaction and a rearrangement reaction named after British chemist Samuel Smiles. It is an intramolecular nucleophilic aromatic substitution of the type: where X in the arene compound can be a sulfone, a sulfide, an ether or any substituent capable of dislodging from the arene carrying a negative charge. The terminal functional group in the chain end Y is able to act as a strong nucleophile for instance an alcohol, amine or thiol. As in other nucleophilic aromatic substitutions the arene requires activation by an electron-withdrawing group preferably in the aromatic ortho position. In one modification called the Truce–Smiles rearrangement the incoming nucleophile is sufficiently strong that the arene does not require this additional activation, for example when the nucleophile is an organolithium. This reaction is exemplified by the conversion of an aryl sulfone into a sulfinic acid by action of ''n''-butyllithium: This particul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Naphthalenethiol
2-Naphthalenethiol is an organosulfur compound with the formula C10H7SH. It is a white solid. It is one of two monothiols of naphthalene, the other being 1-naphthalenethiol. Synthesis and reactions 2-Naphthalenethiol is prepared from 2-naphthol by the Newman–Kwart rearrangement starting from a thiocarbamate In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix ''thio-'' suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: ''O''-thiocarbamates, (ester .... It undergoes lithiation at the 1 and 3-position. It can be used as a flavouring agent. References {{DEFAULTSORT:Naphthalenethiol, 2- Thiols 2-Naphthyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocarbamoyl Chloride
Dimethylthiocarbamoyl chloride is an organosulfur compound with the formula (CH3)2NC(S)Cl. A yellow solid, it is often encountered as a yellow syrup. It is a key reagent in the synthesis of arylthiols via the Newman-Kwart rearrangement. Synthesis and reactions Representative of other thiocarbamoyl chlorides, dimethylthiocarbamoyl chloride is electrophilic, serving as a source of R2NC(S)+.R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). It is analogous to dimethylcarbamoyl chloride (R2NC(O)Cl). Dimethylthiocarbamoyl chloride is prepared by chlorination of the related tetramethylthiuram disulfide: : e2NC(S)sub>2S2 + 3 Cl2 → 2 Me2NC(S)Cl + 2 SCl2 Dimethylthiocarbamoyl chloride reacts with dithiocarbamates (R2NCS{{su, b=2, p=−) to give thiuram sulfides 2NC(S)sub>2S. With methanethiolate, it gives methyl dimethyldithiocarbamate Methyl dimethyldithiocarbamate is the organosulfur compound with the formula (CH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophenols
Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols except the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom. Thiophenols also describes a class of compounds formally derived from thiophenol itself. All have a sulfhydryl group (-SH) covalently bonded to an aromatic ring. The organosulfur ligand in the medicine thiomersal is a thiophenol. Synthesis There are several methods of synthesis for thiophenol and related compounds, although thiophenol itself is usually purchased for laboratory operations. Methods a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |