|
Neutral Fat
Neutral fats, also known as true fats, are simple lipids that are produced by the dehydration synthesis of one or more fatty acids with an alcohol like glycerol. Many types of neutral fats are possible both because of the number and variety of fatty acids that could form part of it and because of the different bonding locations for the fatty acids. An example is a monoglyceride, which has one fatty acid combined with glycerol, a diglyceride, which has two fatty acids combined with glycerol, or a triglyceride A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride''). Triglycerides are the main constituents of body fat in humans and other vertebrates, as w ..., which has three fatty acids combined with glycerol. Triglycerides Triglycerides are formed from the esterification of 3 molecules of fatty acids with one molecule of trihydric alcohol, glycerol (glycerine or trihydroxy propa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Synthesis
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Dehydration reactions in organic chemistry Esterification The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol to give an ester :RCO2H + R′OH RCO2R′ + H2O Often such reactions require the presence of a dehydrating agent, i.e. a substance that reacts with water. Etherification Two monosaccharides, such as glucose and fructose, can be joined together (to form saccharose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide. Nitrile formation Nitriles are often prepared by dehydration of primary amides. :RC(O)NH2 → RCN + H2O Ketene formation Ketene is produced by heating acetic acid and trapping the product: :CH3CO2H → CH2=C= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsaturati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
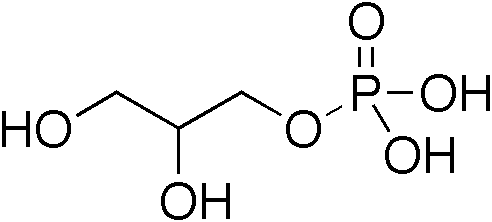
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyceride
Glycerides, more correctly known as acylglycerols, are esters formed from glycerol and fatty acids, and are generally very hydrophobic. Glycerol has three hydroxyl functional groups, which can be esterified with one, two, or three fatty acids to form mono-, di-, and triglycerides. These structures vary in their fatty acid alkyl groups as they can contain different carbon numbers, different degrees of unsaturation, and different configurations and positions of olefins. Vegetable oils and animal fats contain mostly triglycerides, but are broken down by natural enzymes (lipases) into mono and diglycerides and free fatty acids and glycerol. Soaps are formed from the reaction of glycerides with sodium hydroxide. The product of the reaction is glycerol and salts of fatty acids. Fatty acids in the soap emulsify the oils in dirt, enabling the removal of oily dirt with water. Partial glycerides are esters of glycerol with fatty acids, where not all the hydroxyl groups are esterified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diglyceride
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil (particularly 1,3-DAG) has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009. Production Diglycerides are a minor component of many seed oils and are normally present at ~1–6%; or in the case of cottonseed oil as much as 10%. Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol. The raw materials for this may be either vegetable oils or animal fats. Food additive Diglycerides, generally in a mix with monoglycerides (E471), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride''). Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils. Many types of triglycerides exist. One specific classification focuses on saturated and unsaturated types. Saturated fats have ''no'' C=C groups; unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature. Chemical structure Triglycerides are tri-esters consisting of a glycerol bound to three fatty acid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |