|
Neodymium(III) Sulfate
Neodymium(III) sulfate is a salt of the rare-earth metal neodymium that has the formula Nd2(SO4)3. It forms multiple hydrates, the octa-, penta-, and the dihydrate, which the octahydrate is the most common. This compound has a retrograde solubility, unlike other compounds, its solubility decreases with increasing temperature. This compound is used in glass for extremely powerful lasers. Preparation Neodymium sulfate is produced by dissolving neodymium(III) oxide in sulfuric acid: : It can also be prepared by the reaction of neodymium(III) perchlorate and sodium sulfate. Properties Neodymium sulfate octahydrate decomposes at 40 °C to the pentahydrate, which in turn decomposes to the dihydrate at 145 °C. The dihydrate dehydrates to the anhydrous A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium Nitrate
Neodymium nitrate is a chemical compound with the formula Nd(NO3)3. It is typically encountered as the hexahydrate, Nd(NO3)3·6H2O, which is more accurately formulated as d(NO3)3(H2O)42H2O to reflect the crystal structure. It decomposes to NdONO3 at elevated temperature. This water-soluble salt finds use in fabrication of perovskite (CaTiO3) based solid oxide fuel cells, synthesis of Nd3+ doped vanadium pentoxide (V2O5) nanostructure for potential usage in supercapacitors and as a catalyst for Friedlander synthesis of surface modified quinolones Quinolone may refer to: * 2-Quinolone * 4-Quinolone * Quinolone antibiotic A quinolone antibiotic is a member of a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They ar ... for application in medicinal chemistry. References External links Neodymium Nitrate at americanelements.com Neodymium compounds Nitrates {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
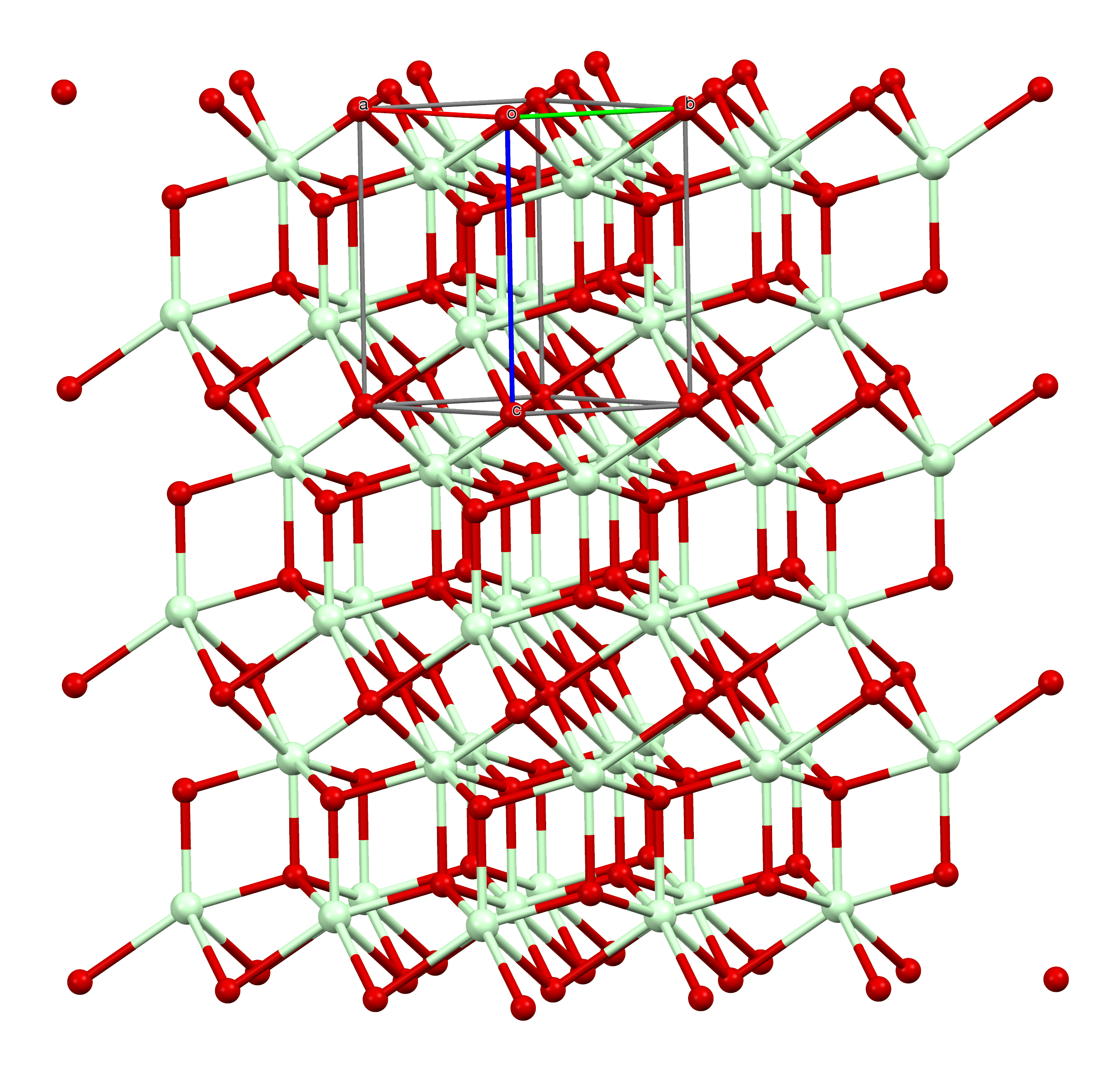
Praseodymium(III) Sulfate
Praseodymium(III) sulfate is a praseodymium compound with formula Pr2(SO4)3. It is an odourless whitish-green crystalline compound. The anhydrous substance readily absorbs water forming pentahydrate and octahydrate. Properties Praseodymium sulfate is stable under standard conditions. At elevated temperatures, it gradually loses water and becomes more whitish. Like all rare earth sulfates, its solubility ''decreases'' with temperature, a property once used to separate it from other, non-rare earth compounds. Pentahydrate and octahydrate have monoclinic crystal structures with densities of 3.713 and 2.813 g/cm3, respectively. The octahydrate crystals are optically biaxial, with refractive index components of nα = 1.5399, nβ = 1.5494 and nγ = 1.5607. They belong to the space group C12/c1 (No. 15) and have lattice constants ''a'' = 1370.0(2) pm, ''b'' = 686.1(1) pm, ''c'' = 1845.3(2) pm, β = 102.80(1)° and ''Z'' = 4. Synthesis Crystals of octahydrate can be grown from soluti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Metal
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The first laser was built in 1960 by Theodore H. Maiman at Hughes Research Laboratories, based on theoretical work by Charles Hard Townes and Arthur Leonard Schawlow. A laser differs from other sources of light in that it emits light which is ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and lithography. Spatial coherence also allows a laser beam to stay narrow over great distances (collimation), enabling applications such as laser pointers and lidar (light detection and ranging). Lasers can also have high temporal coherence, which allows them to emit light with a very narrow spectrum. Alternatively, temporal coherence can be used to produce ultrashort pulses of ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Oxide
Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish-blue hexagonal crystals. The rare-earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide. Uses Neodymium(III) oxide is used to dope glass, including sunglasses, to make solid-state lasers, and to color glasses and enamels. Neodymium-doped glass turns purple due to the absorbance of yellow and green light, and is used in welding goggles. Some neodymium-doped glass is dichroic; that is, it changes color depending on the lighting. One kind of glass named for the mineral alexandrite appears blue in sunlight and red in artificial light. About 7000 tonnes of neodymium(III) oxide are produced worldwide each year. Neodymium(III) oxide is also used as a polymerization catalyst. Reactions Neodymium(III) oxide is formed when neodymium(III) nitride or neodymium(III) hydroxide is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Perchlorate
Neodymium(III) perchlorate is an inorganic compound. It is a salt of neodymium and perchloric acid with the chemical formula of Nd(ClO4)3 – it is soluble in water, forming purple-pink, hydrated crystals. Properties Physical properties Neodymium(III) perchlorate forms pale purple crystals when in its anhydrous form. It is soluble in water. It forms crystals Nd(ClO4)3·''n'' H2O, where ''n'' = 4, 4.5 are purple-pink crystals, ''and n'' = 6 forms pale pink to lavender crystals. Alkaline salts Nd(ClO4)3 can form alkaline salts, with the general formula of Nd(OH)x(ClO4)3 − x. The salt with x = 1.5 (saturated with 5 water atoms) is a light purple crystal with d = 2.88 g/cm³. Other compounds Nd(ClO4)3 can form compounds with hydrazine, such as Nd(ClO4)3·6N2H4·4H2O which is a small white crystal that is soluble in water, methanol, ethanol and acetone, and insoluble in toluene Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides. Forms *Anhydrous sodium sulfate, known as the rare mineral thenardite, used as a drying agent in organic synthesis. *Heptahydrate sodium sulfate, a very rare form. *Decahydrate sodium sulfate, known as the mineral mirabilite, widely used by chemical industry. It is also known as Glauber's salt. History The decahydrate of sodium sulfate is known as Glauber's salt after the Dutch/German chemist and apothecary Johann Rudolf Glauber (1604–1670), who discovered it in Austrian spring water in 1625. He named it (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_chloride.jpg)