|
Neoconvalloside
Neoconvalloside is a cardenolide glycoside In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. ... extracted from '' Convallaria majalis''. References Cardenolides Aldehydes Rhamnosides {{steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardenolide
A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+‐ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes. Etymology The term derives from ''card-'' "heart" (from Greek καρδία ''kardiā'') and the suffix ''-enolide'', referring to the lactone ring at C17. Cardenolides are a class of steroids (or aglycones if viewed as cardiac glycoside constituents), and cardenolides are a subtype of this class (see MeSH D codes list). Structure Cardenolides are C(23)-steroids with methyl groups at C-10 and C-13 and a five-membered lactone (specifically a butenolide) at C-17. They are aglycone constituents o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
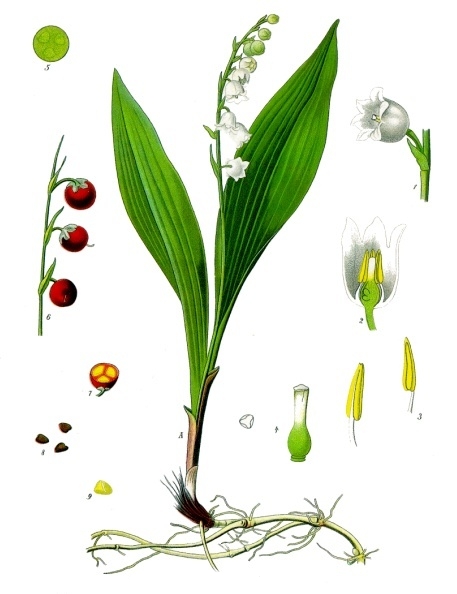
Convallaria Majalis
Lily of the valley (''Convallaria majalis'' (), sometimes written lily-of-the-valley, is a woodland flowering plant with sweetly scented, pendent, bell-shaped white flowers borne in sprays in spring. It is native throughout the cool temperate Northern Hemisphere in Asia and Europe. ''Convallaria majalis'' var. ''montana'', also known as the American lily of the valley, is native to North America. Due to the concentration of cardiac glycosides (cardenolides), it is highly poisonous if consumed by humans or other animals. Other names include May bells, Our Lady's tears, and Mary's tears. Its French name, ''muguet'', sometimes appears in the names of perfumes imitating the flower's scent. In pre-modern England, the plant was known as glovewort (as it was a wort used to create a salve for sore hands), or Apollinaris (according to a legend that it was discovered by Apollo). Description ''Convallaria majalis'' is an herbaceous perennial plant that often forms extensive colonies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardenolides
A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+‐ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes. Etymology The term derives from ''card-'' "heart" (from Greek καρδία ''kardiā'') and the suffix ''-enolide'', referring to the lactone ring at C17. Cardenolides are a class of steroids (or aglycones if viewed as cardiac glycoside constituents), and cardenolides are a subtype of this class (see MeSH D codes list). Structure Cardenolides are C(23)-steroids with methyl groups at C-10 and C-13 and a five-membered lactone (specifically a butenolide) at C-17. They are aglycone constituents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an '' O-glycoside''), N- (a '' glycosylamine''), S-(a '' thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. Accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Alde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |