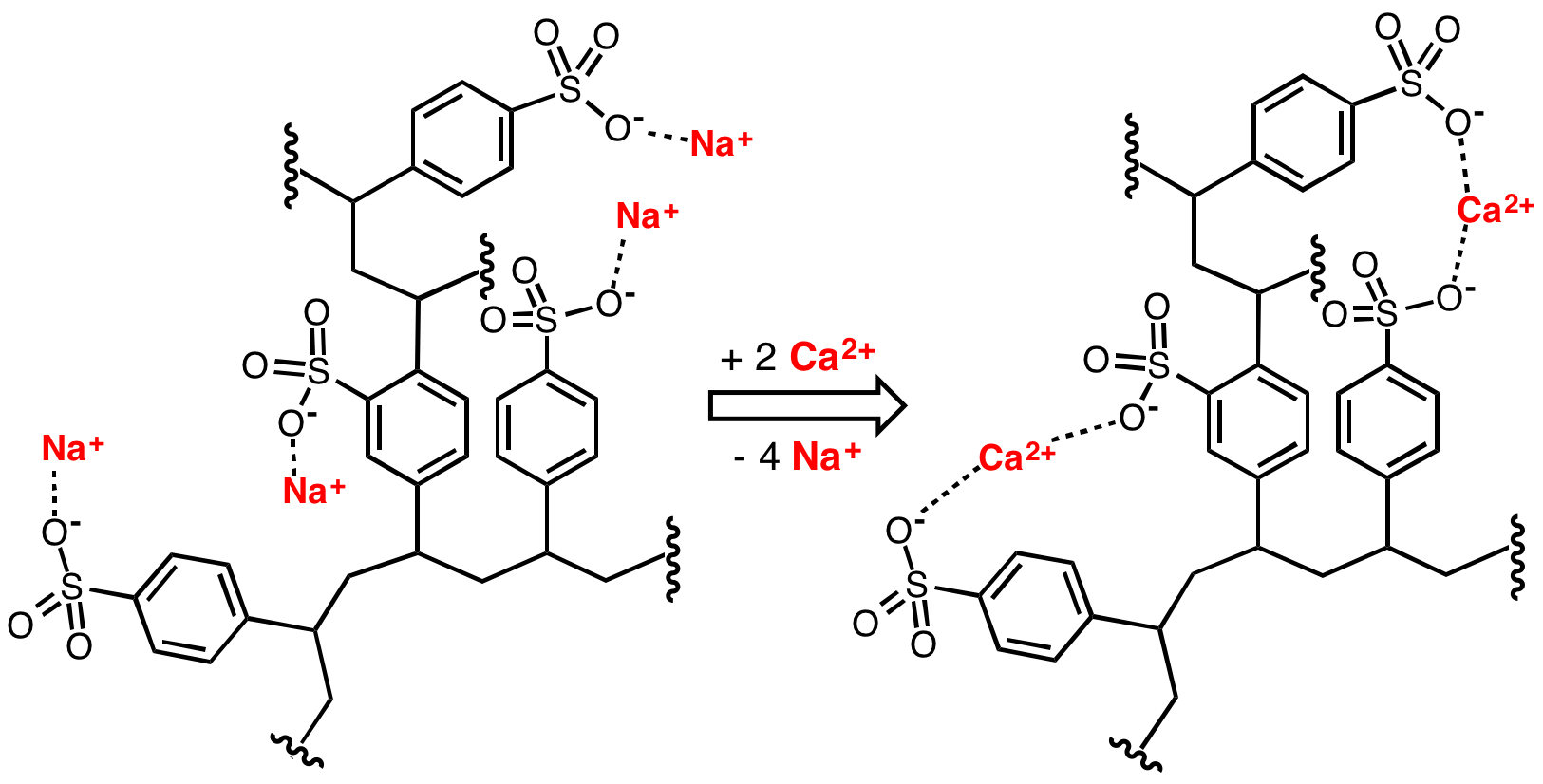
|
Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer- copolymer discovered in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ionic properties that are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone. Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent chemical and mechanical stability in the harsh conditions of this application. The chemical basis of Nafion's ion-conductive properties remain a focus of extensive research. Ion conductivity of Nafion increases with the level of hydration. Exposure of Nafion to a humidified environment or liquid water increases the amount of water molecules associated with each sulfonic acid group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionomer
An ionomer () ('' iono-'' + '' -mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups. The classification of a polymer as an ionomer depends on the level of substitution of ionic groups as well as how the ionic groups are incorporated into the polymer structure. For example, polyelectrolytes also have ionic groups covalently bonded to the polymer backbone, but have a much higher ionic group molar substitution level (usually greater than 80%); ionenes are polymers where ionic groups are part of the actual polymer backbone. These two classes of ionic-group-containing polymers have vastly different morphological and physical properties and are therefore not considered ionomers. Ionomers have unique physical properties including electrical conductivity and v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene ( DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Conductor
A proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers. Composition Acid solutions exhibit proton-conductivity, while pure proton conductors are usually dry solids. Typical materials are polymers or ceramic. Typically, the pores in practical materials are small such that protons dominate direct current and transport of cations or bulk solvent is prevented. Water ice is a common example of a pure proton conductor, albeit a relatively poor one. A special form of water ice, superionic water, has been shown to conduct much more efficiently than normal water ice. Solid-phase proton conduction was first suggested by Alfred Rene Jean Paul Ubbelohde and S. E. Rogers. in 1950, although electrolyte proton currents have been recognized since 1806. Proton conduction has also been observed in the new type of proton conductors for fuel cells – protic organic ionic plastic crystals (POIPCs), such as 1,2,4-triazolium perfluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and ''lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |
Ion Exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances. Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (man-made) resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Abstracts
CAS (formerly Chemical Abstracts Service) is a division of the American Chemical Society. It is a source of chemical information. CAS is located in Columbus, Ohio, United States. Print periodicals ''Chemical Abstracts'' is a periodical index that provides numerous tools such as SciFinder as well as tagged keywords, summaries, indexes of disclosures, and structures of compounds in recently published scientific documents. Approximately 8,000 journals, technical reports, dissertations, conference proceedings, and new books, available in at least 50 different languages, are monitored yearly, as are patent specifications from 27 countries and two international organizations. ''Chemical Abstracts'' ceased print publication on January 1, 2010. Databases The two principal databases that support the different products are CAplus and Registry. CAS References CAS References consists of bibliographic information and abstracts for all articles in chemical journals worldwide, and chemistry-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion-exchange Resins
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate.François Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. . Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification. In many cases ion-exchange resins were introduced in such proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Permeation Chromatography
Gel permeation chromatography (GPC) is a type of size-exclusion chromatography (SEC), that separates analytes on the basis of size, typically in organic solvents. The technique is often used for the analysis of polymers. As a technique, SEC was first developed in 1955 by Lathe and Ruthven.Lathe, G.H.; Ruthven, C.R.J. The Separation of Substance and '1956', ''62'', 665–674. The term ''gel permeation chromatography'' can be traced back to J.C. Moore of the Dow Chemical Company who investigated the technique in 1964. The proprietary column technology was licensed to Waters Corporation, who subsequently commercialized this technology in 1964.Moore, J.C. Gel permeation chromatography. I. A new method for molecular weight distribution of high polymers. ''J. Polym. Sci.'', 1964, ''2'', 835-84 GPC systems and consumables are now also available from a number of manufacturers. It is often necessary to separate polymers, both to analyze them as well as to purify the desired product. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |