|
N2O
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen. Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain-reducing effects. Its colloquial name, "laughing gas", coined by Humphry Davy, is due to the euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is on the World Health Organization's List of Essential Medicines. It is also used as an oxidiser in rocket propellants, and in motor racing to increase the power output of engines. Nitrous oxide's atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of abou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Pentoxide
Dinitrogen pentoxide is the chemical compound with the formula , also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.Peter Steele Connell The Photochemistry of Dinitrogen Pentoxide'. Ph. D. thesis, Lawrence Berkeley National Laboratory. Dinitrogen pentoxide is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by nitronium tetrafluoroborate (). is a rare example of a compound that adopts two structures depending on the conditions. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations and nitrate anions ; but in the gas phase and under some other conditions it is a covalently-bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide *Nitrogen dioxide (), nitrogen(IV) oxide * Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide *Dinitrogen dioxide (), nitrogen(II) oxide dimer *Dinitrogen trioxide (), nitrogen(II,IV) oxide *Dinitrogen tetroxide (), nitrogen(IV) oxide dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate *Nitrosyl azide (), nitrogen(−I,0,I,II) oxide * Nitryl azide () *Oxatetrazole () *Trinitramide ( or ), nitrogen(0,IV) oxide Anions *Nitroxide () * Nitrite ( or ) *Nitrate () *Peroxynitrite ( or ) *Peroxynitrate ( or ) *Orthonitrate (, analogous to phosphate ) *Hyponitrite ( or ) *Trioxodinitrate or hyponitrate ( or ) *Nitroxylate ( or ) * Dinitramide ( or ) Cations * Nitrosonium ( or ) * Nitronium ( or ) Atmospheric sciences In atmospheric chemistry: * (or NO''x'') refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets. Structure and properties Dinitrogen tetroxide could be regarded as two nitro groups (-NO2) bonded together. It forms an equilibrium mixture with nitrogen dioxide. The molecule is planar with an N-N bond distance of 1.78Å and N-O distances of 1.19Å. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.45Å. This exceptionally weak σ bond (amounting to overlapping of the ''sp''2 hybrid orbitals of the two NO2 units) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
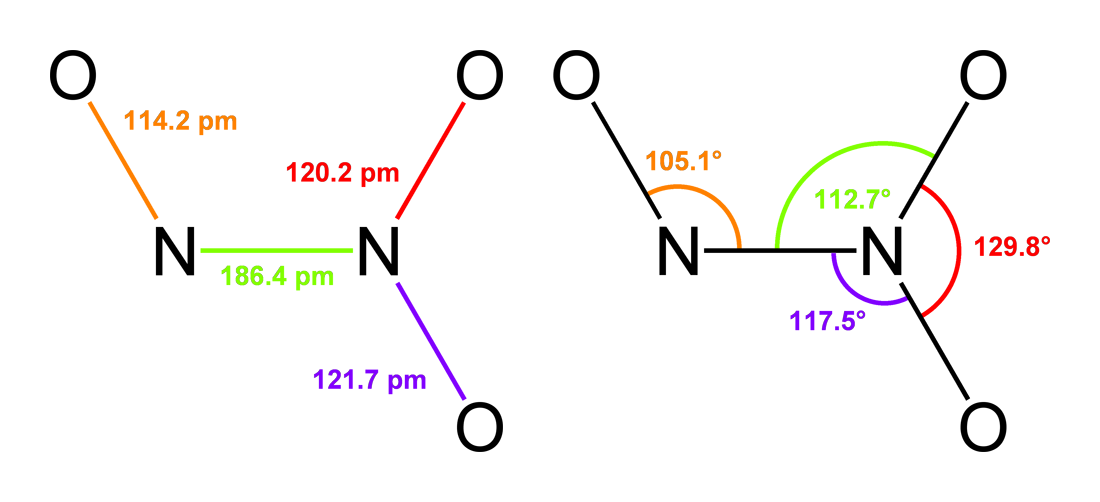
Dinitrogen Trioxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. It is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F): :NO + NO2 N2O3 Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. In liquid and solid states, it has a deep blue color. At higher temperatures the equilibrium favors the constituent gases, with ''K''diss = 193 kPa (25 °C). This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical . Structure and bonding Typically, N–N bonds are similar in length to that in hydrazine (145 pm). Dinitrogen trioxide, however, has an unusually long N–N bond at 186 pm. Some other nitrogen oxides also possess long N–N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry. It is included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry. It is included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Oxide (medication)
Nitrous oxide, is an inhaled gas used as a pain medication and together with other medications for anesthesia. Common uses include during childbirth, following trauma, and as part of end-of-life care. Onset of effect is typically within half a minute, and the effect lasts for about a minute. There are few side effects, other than vomiting, with short-term use. With long-term use anemia or numbness may occur. It should always be given with at least 21% oxygen. It is not recommended in people with a bowel obstruction or pneumothorax. Use in the early part of pregnancy is not recommended. It is possible to continue breastfeeding following use. Nitrous oxide was discovered between 1772 and 1793 and used for anesthesia in 1844. It is on the World Health Organization's List of Essential Medicines. It often comes as a 50/50 mixture with oxygen. Devices with a demand valve are available for self-administration. The setup and maintenance is relatively expensive for developing countrie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flammability
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable material catches fire immediately on exposure to flame. The degree of flammability or combustibility in air depends largely upon the volatility of the material - this is related to its composition-specific vapour pressure, which is temperature dependent. The quantity of vapour produced can be enhanced by increasing the surface area of the material forming a mist or dust. Take wood as an example. Finely divided wood dust can undergo explosive combustion and produce a blast wave. A piece of paper (made from wood) catches on fire quite easily. A heavy oak desk is much harder to ignite, even though the wood fibre is the same in all three materials. Common sense (and indeed scientific consensus until the mid-1700s) would seem to suggest that ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidising Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surgery
Surgery ''cheirourgikē'' (composed of χείρ, "hand", and ἔργον, "work"), via la, chirurgiae, meaning "hand work". is a medical specialty that uses operative manual and instrumental techniques on a person to investigate or treat a pathological condition such as a disease or injury, to help improve bodily function, appearance, or to repair unwanted ruptured areas. The act of performing surgery may be called a surgical procedure, operation, or simply "surgery". In this context, the verb "operate" means to perform surgery. The adjective surgical means pertaining to surgery; e.g. surgical instruments or surgical nurse. The person or subject on which the surgery is performed can be a person or an animal. A surgeon is a person who practices surgery and a surgeon's assistant is a person who practices surgical assistance. A surgical team is made up of the surgeon, the surgeon's assistant, an anaesthetist, a circulating nurse and a surgical technologist. Surgery usually spa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)