|
N-Butylbenzene
''n''-Butylbenzene is the organic compound with the formula C6H5C4H9. Of two isomers of butylbenzene, ''n''-butylbenzene consists of a phenyl group attached to the 1 position of a butyl group. It is a slightly greasy, colorless liquid. The synthesis of ''n''-butylbenzene by the reaction of chlorobenzene and butylmagnesium bromide was one of the first demonstrations of the Kumada coupling using nickel diphosphine Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air. Propert ... catalysts. This mild and efficient process contrasted with older methods. See also * C4-Benzenes References {{Hydrocarbons Alkylbenzenes C4-Benzenes Butyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iso-Butylbenzene
Isobutylbenzene is a chemical compound with the molecular formula C10H14. It is used in the industrial manufacture of ibuprofen Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arte .... Isobutylbenzene is a colorless flammable liquid that is a respiratory irritant. References Alkylbenzenes C4-Benzenes {{Hydrocarbons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sec-Butylbenzene
''sec''-Butylbenzene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring substituted with a ''sec''-butyl group. It is a flammable colorless liquid which is nearly insoluble in water but miscible with organic solvents. Production ''sec''-Butylbenzene can be produced by the reaction of benzene with either ''n''-butyl alcohol or ''sec''-butyl alcohol in presence of anhydrous aluminium chloride and hydrochloric acid Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbol .... References Alkylbenzenes C4-Benzenes {{Hydrocarbons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
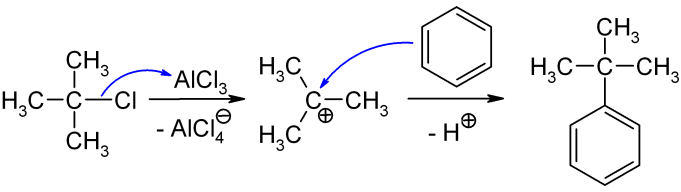
Tert-Butylbenzene
''tert''-Butylbenzene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring substituted with a ''tert''-butyl group. It is a flammable colorless liquid which is nearly insoluble in water but miscible with organic solvents. Production ''tert''-Butylbenzene can be produced by the treatment of benzene with isobutene Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value. Productio ... or by the reaction of benzene with ''tert''-butyl chloride in presence of anhydrous aluminium chloride, the latter is depicted below: References Alkylbenzenes C4-Benzenes {{Hydrocarbons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. Uses Historical The major use of chlorobenzene is as an intermediate in the production of herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in industrial applications as well as in the laboratory. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar. Chlorobenzene once was used in the manufacture of pesticides, most notably DDT, by reaction with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kumada Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki reaction, Suzuki, Sonogashira coupling, Sonogashira, Stille coupling, Stille, Hiyama coupling, Hiyama, Negishi coupling, Negishi), the Kumada coupling continues to be employed in many Chemical synthesis, synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphine
Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air. Properties, preparation, reactions Diphosphane adopts the gauche conformation (like hydrazine, less symmetrical than shown in the image) with a P−P distance of 2.219 angstroms. It is nonbasic, unstable at room temperature, and Pyrophoricity, spontaneously flammable in air. It is only poorly soluble in water but dissolves in organic solvents. Its 1H NMR spectrum consists of 32 lines resulting from an A2XX'A'2 splitting system. Diphosphane is produced by the hydrolysis of calcium monophosphide, which can be described as the Ca2+ derivative of . According to an optimized procedure, hydrolysis of 400 g of CaP at −30 °C gives about 20 g of product, slightly contaminated with phosphine. Reaction of diphosphane with butyllithium affords a varie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylbenzenes
The alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups of different sizes. They are a subset of the aromatic hydrocarbons. The simplest member is toluene, in which a hydrogen atom of the benzene was replaced by a methyl group In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many .... Literature * Allinger, Cava, de Jongh, Johnson, Lebel, Stevens: ''Organische Chemie'', 1. Auflage, Walter de Gruyter, Berlin 1980, , pp. 367–368, 560–562. * Streitwieser / Heathcock: ''Organische Chemie'', 1. Auflage, Verlag Chemie, Weinheim 1980, , pp. 1051, 1073–1080. * Beyer / Walter: ''Lehrbuch der Organischen Chemie'', 19. Auflage, S. Hirzel Verlag, Stuttgart 1981, , pp. 442–444. * Morrison / Boyd: ''Lehrbuch der Organischen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |