|
N-Methylspiperone
''N''-Methylspiperone (NMSP) is a derivate of spiperone that is used to study the dopamine and serotonin neurotransmitter systems. Labeled with the radioisotope carbon-11 Carbon (6C) has 15 known isotopes, from to , of which and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature—trace quantities are formed cosmogenically by ..., it can be used for positron emission tomography. References Dopamine antagonists Piperidines Lactams Fluoroarenes Spiro compounds Aromatic ketones {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiperone
Spiperone (Spiroperidol; brand name: Spiropitan ( JP)) is a typical antipsychotic and research chemical belonging to the butyrophenone chemical class. It is licensed for clinical use in Japan as a treatment for schizophrenia. Additionally, spiperone was identified by compound screening to be an activator of Ca2+ activated Cl− channels (CaCCs), thus a potential target for therapy of cystic fibrosis. ''N''-Methylspiperone (NMSP) is a derivate of spiperone that is used to study the dopamine and serotonin neurotransmitter system. Labeled with the radioisotope carbon-11, it can be used for positron emission tomography. References {{Navboxes , title = Pharmacodynamics Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms ... , titlestyle = background:#ccccff , list1 = {{Adrener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-11
Carbon (6C) has 15 known isotopes, from to , of which and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature—trace quantities are formed cosmogenically by the reaction + → + . The most stable artificial radioisotope is , which has a half-life of . All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is , with a half-life of . List of isotopes , - , , style="text-align:right" , 6 , style="text-align:right" , 2 , , [] , proton emission, 2p , Subsequently decays by double proton emission to for a net reaction of → + 4 , 0+ , , , - , rowspan=3, , rowspan=3 style="text-align:right" , 6 , rowspan=3 style="text-align:right" , 3 , rowspan=3, , rowspan=3, , β+ () , , rowspan=3, 3/2− , rowspan=3, , rowspan=3, , - , β+α () , Immediately decays by proton emission to for a net reaction of � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
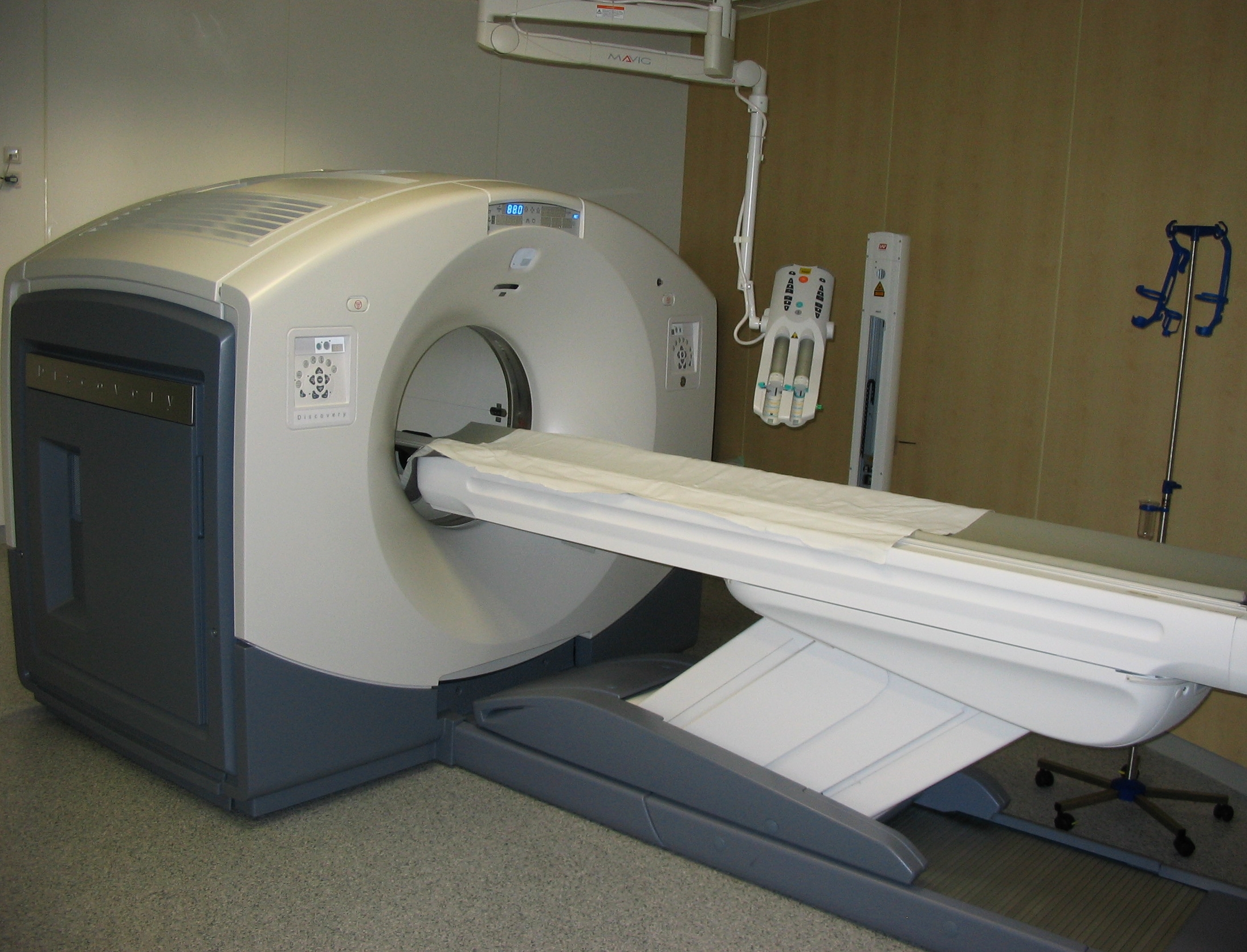
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, -FDG is commonly used to detect cancer, NaF is widely used for detecting bone formation, and oxygen-15 is sometimes used to measure blood flow. PET is a common imaging technique, a medical scintillography technique used in nuclear medicine. A radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is emitted, and when the positron collides with an ordinary electron, the two particles annihilate and gamma rays are emitted. These gamma rays are detecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Clinical Psychopharmacology
The ''Journal of Clinical Psychopharmacology'' is a peer-reviewed medical journal published by Lippincott Williams & Wilkins covering clinical psychopharmacology. It was founded by Richard I. Shader, MD in 1981 as the first journal of an international scope devoted solely to clinical psychopharmacology. David J. Greenblatt, MD served as Co-Editor-In-Chief. Drs. Shader and Greenblatt remained at the helm of the journal until both retired at the end of 2020. Anthony J. Rothschild, MD became JCP's new Editor-in-Chief in January 2021. The first issue of the journal was published in January 1981. It included articles on several psychopharmacological studies, review articles, a brief report of clinicians' observations, an abstract, a historical perspective article, a book review, a forensic update, and a “Question the Experts” section. According to the first editorial by the Editors-in-Chief, this format was chosen “to create a “one stop” journal in which clinicians and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Antagonists
A dopamine antagonist, also known as an anti-dopaminergic and a dopamine receptor antagonist (DRA), is a type of drug which blocks dopamine receptors by receptor antagonism. Most antipsychotics are dopamine antagonists, and as such they have found use in treating schizophrenia, bipolar disorder, and stimulant psychosis. Several other dopamine antagonists are antiemetics used in the treatment of nausea and vomiting. Receptor pharmacology Dopamine receptors are all G protein–coupled receptors, and are divided into two classes based on which G-protein they are coupled to. The D1-like class of dopamine receptors is coupled to Gαs/olf and stimulates adenylate cyclase production, whereas the D2-like class is coupled to Gαi/o and thus inhibits adenylate cyclase production. D1-like receptors: D1 and D5 D1-like receptors – D1 and D5 are always found post-synaptically. The genes coding these receptors lack introns, so there are no splice variants. D1 receptors * D1 receptors ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name '' Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words '' lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size: * α-Lactam (3-atom rings) * β-Lactam (4-atom rings) * γ-Lactam (5-atom rings) * δ-Lactam (6-atom rings) * ε-Lactam (7-atom rings) This ring-size nomenclature stems from the fact that a hydrolyzed α-Lactam leads to an α-amino acid and a β-Lactam to a β-amino acid, ''etc''. Synthesis General synthetic methods exist for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compounds
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |