|
Myron L. Bender
Myron Lee Bender (1924–1988) was born in St. Louis, Missouri. He obtained his B.S. (1944) and his Ph.D. (1948) from Purdue University. The latter was under the direction of Henry B. Hass. After postdoctoral research under Paul D. Barlett (Harvard University), and Frank H. Westheimer (University of Chicago), he spent one year as a faculty member at the University of Connecticut. Thereafter, he was a professor of Chemistry at Illinois Institute of Technology in 1951, and then at Northwestern University in 1960. He worked primarily in the study of reaction mechanisms and the biochemistry of enzyme action. Myron L. Bender demonstrated the two-step mechanism of catalysis for serine proteases, nucleophilic catalysis in ester hydrolysis and intramolecular catalysis in water. He also showed that cyclodextrin can be used to investigate catalysis of organic reactions within the scope of host–guest chemistry. Finally, he and others reported on the synthesis of an organic compound as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purdue University
Purdue University is a public land-grant research university in West Lafayette, Indiana, and the flagship campus of the Purdue University system. The university was founded in 1869 after Lafayette businessman John Purdue donated land and money to establish a college of science, technology, and agriculture in his name. The first classes were held on September 16, 1874, with six instructors and 39 students. It has been ranked as among the best public universities in the United States by major institutional rankings, and is renowned for its engineering program. The main campus in West Lafayette offers more than 200 majors for undergraduates, over 70 masters and doctoral programs, and professional degrees in pharmacy, veterinary medicine, and doctor of nursing practice. In addition, Purdue has 18 intercollegiate sports teams and more than 900 student organizations. Purdue is the founding member of the Big Ten Conference and enrolls the largest student body of any individual univer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Northwestern University
Northwestern University is a private research university in Evanston, Illinois. Founded in 1851, Northwestern is the oldest chartered university in Illinois and is ranked among the most prestigious academic institutions in the world. Chartered by the Illinois General Assembly in 1851, Northwestern was established to serve the former Northwest Territory. The university was initially affiliated with the Methodist Episcopal Church but later became non-sectarian. By 1900, the university was the third largest university in the United States. In 1896, Northwestern became a founding member of the Big Ten Conference, and joined the Association of American Universities as an early member in 1917. The university is composed of eleven undergraduate, graduate, and professional schools, which include the Kellogg School of Management, the Pritzker School of Law, the Feinberg School of Medicine, the Weinberg College of Arts and Sciences, the Bienen School of Music, the McCormick ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JoAnne Stubbe
JoAnne Stubbe is an American chemist best known for her work on ribonucleotide reductases, for which she was awarded the National Medal of Science in 2009. In 2017, she retired as a Professor of Chemistry and Biology at the Massachusetts Institute of Technology. Career and education In 1946, Stubbe was born in Champaign, Illinois. In 1968, Stubbe received a BS degree in chemistry from the University of Pennsylvania, and worked as an undergraduate in the laboratory of Professor Edward R. Thornton. After she received her PhD degree in organic chemistry under the guidance of Professor George Kenyon from the University of California, Berkeley in 1971, she did a very brief stint (1971-1972) as a postdoc at UCLA, where she worked on synthesizing LSD from tryptophan with Julius Rebek. Then, Stubbe taught at Williams College (1972-1977) discovered she didn't want to teach, but wanted to do research. Her realization sent her to Brandeis University (1975-1977), where she did a second post ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Julius Rebek
Julius Rebek Jr. (born Gyula Rebek on April 11, 1944) is a Hungarian-American chemist and expert on molecular self-assembly. Rebek was born in Beregszász (present-day Berehove, Ukraine), which at the time was part of Hungary, in 1944 and lived in Austria from 1945 to 1949. In 1949 he and his family immigrated to the United States and settled in Topeka, Kansas where he graduated from Highland Park High School. Rebek graduated from the University of Kansas with a Bachelor of Arts degree in chemistry. Rebek received his Master of Arts degree and his Ph.D. in organic chemistry from the Massachusetts Institute of Technology in 1970. There he studied peptides under D. S. Kemp. Rebek was an assistant professor at the University of California at Los Angeles from 1970 to 1976. There he developed the three-phase test for reactive intermediates. In 1976, he moved to the University of Pittsburgh, where he developed cleft-like structures for studies in molecular recognition. In 1989 he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUBMB
The International Union of Biochemistry and Molecular Biology (IUBMB) is an international non-governmental organisation concerned with biochemistry and molecular biology. Formed in 1955 as the International Union of Biochemistry (IUB), the union has presently 79 member countries and regions (as of 2020).IUBMB: the first half-century. /ref> The Union is devoted to promoting research and education in biochemistry and molecular biology throughout the world and gives particular attention to areas where the subject is still in its early development History The first Congress of Biochemistry ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michaelis–Menten Kinetics
In biochemistry, Michaelis–Menten kinetics is one of the best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate v (rate of formation of product, ce P/math>) to ce S/math>, the concentration of a substrate ''S''. Its formula is given by : v = \frac = V_\max \frac This equation is called the Michaelis–Menten equation. Here, V_\max represents the maximum rate achieved by the system, happening at saturating substrate concentration for a given enzyme concentration. When the value of the Michaelis constant K_\mathrm is numerically equal to the substrate concentration, then the reaction rate is half of V_\max. Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions. Model In 1901, French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subtilisin
Subtilisin is a protease (a protein-digesting enzyme) initially obtained from ''Bacillus subtilis''. Subtilisins belong to subtilases, a group of serine proteases that – like all serine proteases – initiate the nucleophilic attack on the peptide (amide) bond through a serine residue at the active site. Subtilisins typically have molecular weights 27kDa. They can be obtained from certain types of soil bacteria, for example, ''Bacillus amyloliquefaciens'' from which they are secreted in large amounts. Nomenclature Subtilisin is also commercially known as ''Alcalase®'', ''Endocut-02L'', ''ALK-enzyme'', ''bacillopeptidase'', ''Bacillus subtilis alkaline proteinase bioprase'', ''bioprase AL'', ''colistinase'', ''genenase I'', ''Esperase®'', ''maxatase'', ''protease XXVII'', ''thermoase'', ''superase'', ''subtilisin DY'', ''subtilopeptidase'', ''SP 266'', ''Savinase®'', ''kazusase'', ''protease VIII'', ''protin A 3L'', ''Savinase®'', ''orientase 10B'', ''protease S.'' It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies on carboxypeptidases focused on pancreatic carboxypeptidases A1, A2, and B in the digestion of food. Most carboxypeptidases are not, however, involved in catabolism. Instead they help to mature proteins, for example Post-translational modification. They also regulate biological processes, such as the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, reproduction, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
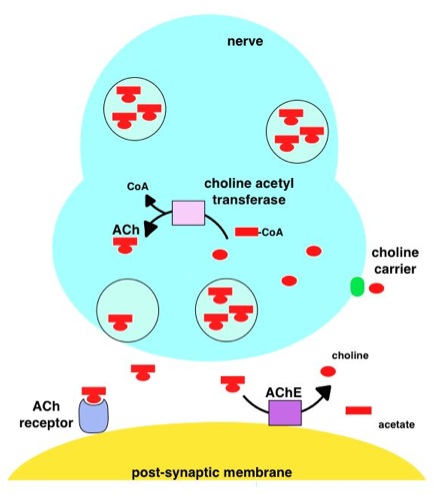
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ... that catalysis, catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate neurotransmission, synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chymotrypsin
Chymotrypsin (, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly tho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States National Academy Of Sciences
The National Academy of Sciences (NAS) is a United States nonprofit, non-governmental organization. NAS is part of the National Academies of Sciences, Engineering, and Medicine, along with the National Academy of Engineering (NAE) and the National Academy of Medicine (NAM). As a national academy, new members of the organization are elected annually by current members, based on their distinguished and continuing achievements in original research. Election to the National Academy is one of the highest honors in the scientific field. Members of the National Academy of Sciences serve '' pro bono'' as "advisers to the nation" on science, engineering, and medicine. The group holds a congressional charter under Title 36 of the United States Code. Founded in 1863 as a result of an Act of Congress that was approved by Abraham Lincoln, the NAS is charged with "providing independent, objective advice to the nation on matters related to science and technology. ... to provide scie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Host–guest Chemistry
In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. Although non-covalent interactions could be roughly divided into those with more electrostatic or dispersive contributions, there are few commonly mentioned types of non-covalent interactions: ionic bonding, hydrogen bonding, van der Waals forces and hydrophobic interactions. Overview Host–guest chemistry is a branch of supramolecular chemistry in which a host molecule forms a chemical compound with a guest molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |