|
Murai Reaction
In organic chemistry, the Murai reaction is an organic reaction that uses C-H activation to create a new C-C bond between a terminal or strained internal alkene and an aromatic compound using a ruthenium catalyst. The reaction, named after Shinji Murai, was first reported in 1993. While not the first example of C-H activation, the Murai reaction is notable for its high efficiency and scope. Previous examples of such hydroarylations required more forcing conditions and narrow scope. Scope and regiochemistry The reaction was initially demonstrated using a ketone as the directing group, but other functional groups have been reported, including esters, imines, nitriles, and imidates. Murai reactions have also been reported with disubstituted alkynes. bidentate directing group allow ''ortho'' alkylation of aromatic rings with α,β-unsaturated ketones, which typically are unreactive in Murai reactions. Early examples of the reaction suffered from side products of alkylation at bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
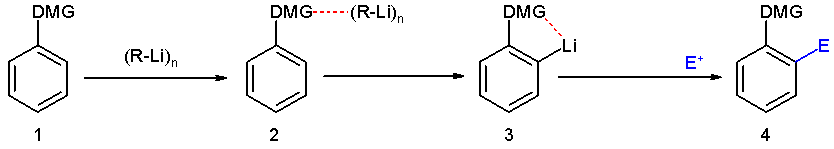
Directing Group
In organic chemistry, a directing group (DG) is a substituent on a molecule or ion that facilitates reactions by interacting with a reagent. The term is usually applied to C-H activation of hydrocarbons, where it is defined as a "coordinating moiety (an “internal ligand”), which directs a metal catalyst into the proximity of a certain C–H bond." In a well known example, the ketone group () in acetophenone is the DG in the Murai reaction. The Murai reaction is related to directed ortho metalation, a reaction is typically applied to the lithiation of substituted aromatic rings.''Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics Victor Snieckus'' Chem. Rev.; 1990; 90(6); 879-933Abstract/ref> A wide variety of functional groups can serve as directing groups. Transient directing groups Since directing groups are ligands, their effectiveness correlates with their affinities for metals. Common fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroarylation
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene (). The more general reaction, hydroalkenylation, is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene: :\ce + \longrightarrow \ce Ethylene dimerization The dimerization of ethylene gives 1-butene is another example of a hydrovinylation. In the Dimersol and Alphabutol Processes, alkenes are dimerized for the production of gasoline and for comonomers such as 1-butene. These processes operate at several refineries across the world at the scales of about 400,000 tons/year (2006 report). 1-Butene is amenable to isomerization to 2-butenes, which is used in Olefin conversion technology to give propylene. Hydroarylation Hydroarylation is again a special case of hydrovinylation. Hydroarylation has been demonstrated for alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agostic Interaction
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, resulting in a three-center two-electron bond. Many catalytic transformations, e.g. oxidative addition and reductive elimination, are proposed to proceed via intermediates featuring agostic interactions. Agostic interactions are observed throughout organometallic chemistry in alkyl, alkylidene, and polyenyl ligands. History The term agostic, derived from the Ancient Greek word for "to hold close to oneself", was coined by Maurice Brookhart and Malcolm Green, on the suggestion of the classicist Jasper Griffin, to describe this and many other interactions between a transition metal and a C−H bond. Often such agostic interactions involve alkyl or aryl groups that are held close to the metal center through an additional σ-bond.. Short ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals. Nomenclature Typically, metallacycles are cyclic compounds with two metal carbon bonds. Many compounds containing metals in rings are known, for example chelate rings. Usually, such compounds are not classified as metallacycles, but the naming conventions are not rigidly followed. Within the area of coordination chemistry and supramolecular chemistry, examples include metallacrowns, metallacryptands, metallah ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Figure 2
Figure may refer to: General *A shape, drawing, depiction, or geometric configuration *Figure (wood), wood appearance *Figure (music), distinguished from musical motif *Noise figure, in telecommunication *Dance figure, an elementary dance pattern *A person's figure, human physical appearance Arts *Figurine, a miniature statuette representation of a creature *Action figure, a posable jointed solid plastic character figurine *Figure painting, realistic representation, especially of the human form *Figure drawing *Model figure, a scale model of a creature Writing *figure, in writing, a type of floating block (text, table, or graphic separate from the main text) *Figure of speech, also called a rhetorical figure *Christ figure, a type of character * in typesetting, text figures and lining figures Accounting *Figure, a synonym for number *Significant figures in a decimal number Science *Figure of the Earth, the size and shape of the Earth in geodesy Sports *Figure (horse), a st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density Functional Theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals, i.e. functions of another function. In the case of DFT, these are functionals of the spatially dependent electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry. DFT has been very popular for calculations in solid-state physics since the 1970s. However, DFT was not considered accurate enough for calculations in quantum chemistry until the 1990s, when the approximations used in the theory were greatly refined to better model the exchange and correlation interactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triruthenium Dodecacarbonyl
Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds. Structure and synthesis The cluster has ''D3h'' symmetry, consisting of an equilateral triangle of Ru atoms, each of which bears two axial and two equatorial CO ligands. Os3(CO)12 has the same structure, whereas Fe3(CO)12 is different, with two bridging CO ligands, resulting in C2v symmetry. Ru3(CO)12 is prepared by treating solutions of ruthenium trichloride with carbon monoxide in the presence of a base. Dichlororuthenium tricarbonyl dimer is an intermediate. The stoichiometry of the reaction is uncertain, one possibility being the following: :6 RuCl3 + 33 CO + 18 CH3OH → 2 Ru3(CO)12 + 9 CO(OCH3)2 + 18 HCl Reactions The chemical properties of Ru3(CO)12 have been widely studied, and the cluster ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarbonyltris(triphenylphosphine)ruthenium(0)
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center. In solution, this compound readily dissociates one of the three phosphine ligands, thereby generating a reactive 16-electron complex that binds or oxidatively adds a variety of substrates such as alkynes, olefins, dihydrogen, and dioxygen. The compound has a trigonal bipyramidal molecular geometry and, in solution, exists as a mixture of two isomers that rapidly interconvert. The complex is air stable as a solid, but its solutions oxygenate in air to afford Ru(CO)2(PPh3)2(η2-O2). Preparation The compound can be prepared by magnesium reduction of the corresponding ruthenium(II) dichloride complex in the presence of an excess of phosphine. The 16-electron intermediate can actually be isolated. :Ru(CO)2Cl2(PPh3)2 + Mg + PPh3 → Ru(CO)2(PPh3)3 + MgCl2 An improved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)

