|
Mott–Bethe Formula
The Mott–Bethe formula is an approximation used to calculate atomic electron scattering form factors, f_e (q,Z), from atomic X-ray scattering form factors, f_x(q,Z). The formula was derived independently by Hans Bethe and Neville Mott both in 1930, and simply follows from applying the first Born approximation for the scattering of electrons via the Coulomb interaction together with the Poisson equation for the charge density of an atom (including both the nucleus and electron cloud) in the Fourier domain. Following the first Born approximation, :f_e(q,Z)=\frac\Bigg(\frac\Bigg) =\frac\Bigg(\frac\Bigg) \approx (0.2393 \textrm^)\cdot \Bigg(\frac\Bigg) Here, q is the magnitude of the scattering vector of momentum-transfer cross section in reciprocal space (in units of inverse distance), Z the atomic number of the atom, \hbar is Planck's constant, \epsilon_0 is the vacuum permittivity, and m_0 is the electron rest mass, a_0 is the Bohr Radius, and f_x(q,Z) is the dimensionless X-ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
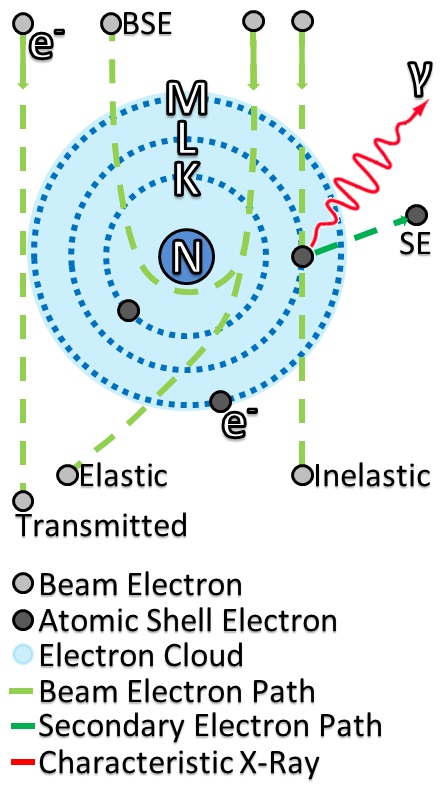
Electron Scattering
Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors. The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks. Electrons may be scattered through a solid in several ways: *Not at all: no electron scattering occurs at all and the beam passes straight through. *Single scattering: when an electron is scattered just once. *Plu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hartree–Fock Method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The Hartree–Fock method often assumes that the exact ''N''-body wave function of the system can be approximated by a single Slater determinant (in the case where the particles are fermions) or by a single permanent (in the case of bosons) of ''N'' spin-orbitals. By invoking the variational method, one can derive a set of ''N''-coupled equations for the ''N'' spin orbitals. A solution of these equations yields the Hartree–Fock wave function and energy of the system. Especially in the older literature, the Hartree–Fock method is also called the self-consistent field method (SCF). In deriving what is now called the Hartree equation as an approximate solution of the Schrödinger equation, Hartree required the final field as computed from the charge distribution to be "s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Classical Electron Radius
The classical electron radius is a combination of fundamental physical quantities that define a length scale for problems involving an electron interacting with electromagnetic radiation. It links the classical electrostatic self-interaction energy of a homogeneous charge distribution to the electron's relativistic mass–energy. According to modern understanding, the electron is a point particle with a point charge and no spatial extent. Nevertheless, it is useful to define a length that characterizes electron interactions in atomic-scale problems. The classical electron radius is given as :r_\text = \frac\frac = 2.817 940 3227(19) \times 10^ \text = 2.817 940 3227(19) \text , where e is the elementary charge, m_ is the electron mass, c is the speed of light, and \varepsilon_0 is the permittivity of free space. This numerical value is several times larger than the radius of the proton. In cgs units, the permittivity factor and \frac do not enter, but the classical electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thomson Cross Section
Thomson scattering is the elastic scattering of electromagnetic radiation by a free charged particle, as described by classical electromagnetism. It is the low-energy limit of Compton scattering: the particle's kinetic energy and photon frequency do not change as a result of the scattering. This limit is valid as long as the photon energy is much smaller than the mass energy of the particle: \nu\ll mc^2/h , or equivalently, if the wavelength of the light is much greater than the Compton wavelength of the particle (e.g., for electrons, longer wavelengths than hard x-rays). Description of the phenomenon In the low-energy limit, the electric field of the incident wave (photon) accelerates the charged particle, causing it, in turn, to emit radiation at the same frequency as the incident wave, and thus the wave is scattered. Thomson scattering is an important phenomenon in plasma physics and was first explained by the physicist J. J. Thomson. As long as the motion of the particle is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Scattering Factor
In physics, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident radiation, typically X-ray, electron or neutron. The common feature of all form factors is that they involve a Fourier transform of a spatial density distribution of the scattering object from real space to momentum space (also known as reciprocal space). For an object with spatial density distribution, \rho(\mathbf), the form factor, f(\mathbf), is defined as f(\mathbf)=\int \rho(\mathbf) e^\mathrm^3\mathbf, where \rho(\mathbf) is the spatial density of the scatterer about its center of mass (\mathbf=0), and \mathbf is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer \rho in real space \mathbf, the narrower the distribution of f in \mathbf; i.e., the faster the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohr Radius
The Bohr radius (''a''0) is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom. Its value is The number in parenthesis denotes the uncertainty of the last digits. Definition and value The Bohr radius is defined as a_0 = \frac = \frac = \frac , where * \varepsilon_0 is the permittivity of free space, * \hbar is the reduced Planck constant, * m_ is the mass of an electron, * e is the elementary charge, * c is the speed of light in vacuum, and * \alpha is the fine-structure constant. The CODATA value of the Bohr radius (in SI units) is History In the Bohr model for atomic structure, put forward by Niels Bohr in 1913, electrons orbit a central nucleus under electrostatic attraction. The original derivation posited that electrons have orbital angular momentum in integer multiples of the reduced Planck co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Mass
The electron mass (symbol: ''m''e) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about or about , which has an energy-equivalent of about or about Terminology The term "rest mass" is sometimes used because in special relativity the mass of an object can be said to increase in a frame of reference that is moving relative to that object (or if the object is moving in a given frame of reference). Most practical measurements are carried out on moving electrons. If the electron is moving at a relativistic velocity, any measurement must use the correct expression for mass. Such correction becomes substantial for electrons accelerated by voltages of over . For example, the relativistic expression for the total energy, ''E'', of an electron moving at speed v is :E = \gamma m_\text c^2 , where the Lorentz factor is \gamma = 1/\sqrt . In this expression ''m''e is the "re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permittivity
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ''ε'' ( epsilon), is a measure of the electric polarizability of a dielectric. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the material. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor. In the simplest case, the electric displacement field D resulting from an applied electric field E is :\mathbf = \varepsilon \mathbf. More generally, the permittivity is a thermodynamic function of state. It can depend on the frequency, magnitude, and direction of the applied field. The SI unit for permittivity is farad per meter (F/m). The permittivity is often represented by the relative permittivity ''ε''r which is the ratio of the absolute permittivity ''ε'' and the vacuum permittivity ''ε''0 :\kappa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number ''A''. Atoms with the same atomic number but dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Form Factor
In physics, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident radiation, typically X-ray, electron or neutron. The common feature of all form factors is that they involve a Fourier transform of a spatial density distribution of the scattering object from real space to momentum space (also known as reciprocal space). For an object with spatial density distribution, \rho(\mathbf), the form factor, f(\mathbf), is defined as f(\mathbf)=\int \rho(\mathbf) e^\mathrm^3\mathbf, where \rho(\mathbf) is the spatial density of the scatterer about its center of mass (\mathbf=0), and \mathbf is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer \rho in real space \mathbf, the narrower the distribution of f in \mathbf; i.e., the faster the dec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reciprocal Space
In physics, the reciprocal lattice represents the Fourier transform of another lattice (usually a Bravais lattice). In normal usage, the initial lattice (whose transform is represented by the reciprocal lattice) is usually a periodic spatial function in real-space and is also known as the ''direct lattice''. While the direct lattice exists in real-space and is what one would commonly understand as a physical lattice (e.g., a lattice of a crystal), the reciprocal lattice exists in reciprocal space (also known as ''momentum space'' or less commonly as ''K-space'', due to the relationship between the Pontryagin duals momentum and position). The reciprocal lattice of a reciprocal lattice is equivalent to the original direct lattice, because the defining equations are symmetrical with respect to the vectors in real and reciprocal space. Mathematically, direct and reciprocal lattice vectors represent covariant and contravariant vectors, respectively. The reciprocal lattice is the se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |