|
Monosodium Citrate
Monosodium citrate, more correctly, sodium dihydrogen citrate (Latin: ''natrium citricum acidulatum''), is an acid salt of citric acid. Disodium citrate and trisodium citrate are also known. It can be prepared by partial neutralisation of citric acid with an aqueous solution of sodium bicarbonate or carbonate. It has a slightly acidic taste. :NaHCO3 + C6H8O7 → NaC6H7O7 + CO2 + H2O :Na2CO3 + 2C6H8O7 → 2NaC6H7O7 + CO2 + H2O It is highly soluble in water and practically insoluble in ethanol. Monosodium citrate is used as an anticoagulant in donated blood. It is used as an alkalinizing agent Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to kidney failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizin ... to prevent kidney stone disease. The crystals form as nearly perfect cubes. References Organic sodium salts Food additives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Salt
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A ''half-neutralization'' occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions () to create water molecules. Acidic solution and examples of acid salts Acid-base property of the resulting solution from a neutralization reaction depends on the remaining salt products. A salt containing reactive cations undergo hydrolysis by which they react with water molecules, causing deprotonation of the conjugate acids. For example, the acid salt ammonium chloride is the main species formed upon the ''half neutralization'' of ammonia in hydrochloric acid solution: :NH3_\ +\ HCl_ -> NH4Cl_ Use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium Citrate
Disodium citrate, also known as disodium hydrogen citrate, Alkacitron, and sesquihydrate, is an acid salt of citric acid with the chemical formula Na2C6H6O7. Uses Food It is used as an antioxidant in food and to improve the effects of other antioxidants. It is also used as an acidity regulator and sequestrant. Typical products include gelatin, jam, sweets, ice cream, carbonated beverages, milk powder, wine, and processed cheese Processed cheese (also known as process cheese, cheese food, prepared cheese, cheese product, or plastic cheese) is a food product made from cheese and unfermented dairy ingredients mixed with emulsifiers. Additional ingredients, such as vegeta ...s. Disodium citrate can also be used as a thickening agent or stabilizer. Manufacturing Disodium citrate can also be used as an ingredient in household products that remove stains. Health Disodium citrate may be used in patients to alleviate discomfort from urinary-tract infections. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trisodium Citrate
Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as "sodium citrate", though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor, and is a mild alkali. Applications Foods Sodium citrate is chiefly used as a food additive, usually for flavor or as a preservative. Its E number is E331. Sodium citrate is employed as a flavoring agent in certain varieties of club soda. It is common as an ingredient in bratwurst, and is also used in commercial ready-to-drink beverages and drink mixes, contributing a tart flavor. It is found in gelatin mix, ice cream, yogurt, jams, sweets, milk powder, processed cheeses, carbonated beverages, and wine, amongst others. Sodium citrate can be used as an emulsifying stabilizer when making cheese. It allows the cheese to melt without becoming greasy by stopping the fats from separating. Buffering As a conjugate base of a weak acid, citrate can per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs. Nomenclature Because it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, and bicarbonate of soda and can often be found near baking powder in stores. The term ''baking soda'' is more common in the United States, while ''bicarbonate of soda'' is more common in Australia, United Kingdom and Ireland. and in many northern/central European countries it is called ''Na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticoagulant
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide. Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation. Specifically, antiplatelet drugs inhibit platelet agg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Donation
A blood donation occurs when a person voluntarily has blood drawn and used for blood transfusion, transfusions and/or made into biopharmaceutical medications by a process called Blood fractionation, fractionation (separation of whole blood components). Donation may be of whole blood, or of specific components directly (apheresis). Blood banks often participate in the collection process as well as the procedures that follow it. Today in the developed world, most blood donors are unpaid volunteers who donate blood for a community supply. In some countries, established supplies are limited and donors usually give blood when family or friends need a transfusion (directed donation). Many donors donate for several reasons, such as a form of charity, general awareness regarding the demand for blood, increased confidence in oneself, helping a personal friend or relative, and social pressure. Despite the many reasons that people donate, not enough potential donors actively donate. Ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinizing Agent
Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to kidney failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizing agent. Others include potassium citrate, calcium carbonate, sodium lactate and calcium acetate Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous .... References {{treatment-stub Drugs acting on the genito-urinary system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
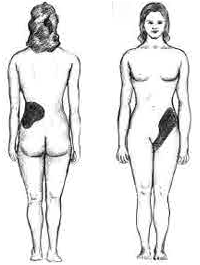
Kidney Stone Disease
Kidney stone disease, also known as nephrolithiasis or urolithiasis, is a crystallopathy where a solid piece of material (kidney stone) develops in the urinary tract. Kidney stones typically form in the kidney and leave the body in the urine stream. A small stone may pass without causing symptoms. If a stone grows to more than , it can cause blockage of the ureter, resulting in sharp and severe pain in the lower back or abdomen. A stone may also result in blood in the urine, vomiting, or painful urination. About half of people who have had a kidney stone will have another within ten years. Most stones form by a combination of genetics and environmental factors. Risk factors include high urine calcium levels, obesity, certain foods, some medications, calcium supplements, hyperparathyroidism, gout and not drinking enough fluids. Stones form in the kidney when minerals in urine are at high concentration. The diagnosis is usually based on symptoms, urine testing, and medical i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |