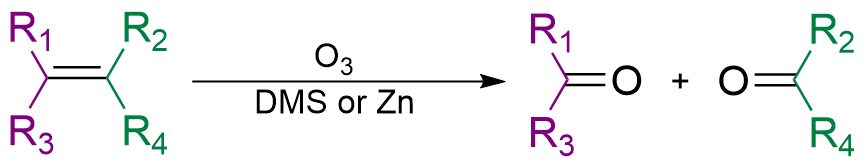|
Molozonide
A molozonide (or "molecular ozonide") is a 1,2,3-trioxolane, which can also be thought of a cyclic dialkyl trioxidane. Molozonides are formed by cycloaddition of ozone and an alkene during ozonolysis, as a transient intermediate which quickly rearranges to give the ozonide Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides. Ionic ozonides Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule. Inor ... (1,2,4-trioxolane), the relatively stable product generated immediately prior to reductive or oxidative cleavage to form alcohols, carbonyl compounds, or derivatives thereof. References Oxygen heterocycles Polyoxides Heterocyclic compounds with 1 ring {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molozonide
A molozonide (or "molecular ozonide") is a 1,2,3-trioxolane, which can also be thought of a cyclic dialkyl trioxidane. Molozonides are formed by cycloaddition of ozone and an alkene during ozonolysis, as a transient intermediate which quickly rearranges to give the ozonide Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides. Ionic ozonides Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule. Inor ... (1,2,4-trioxolane), the relatively stable product generated immediately prior to reductive or oxidative cleavage to form alcohols, carbonyl compounds, or derivatives thereof. References Oxygen heterocycles Polyoxides Heterocyclic compounds with 1 ring {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxygen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
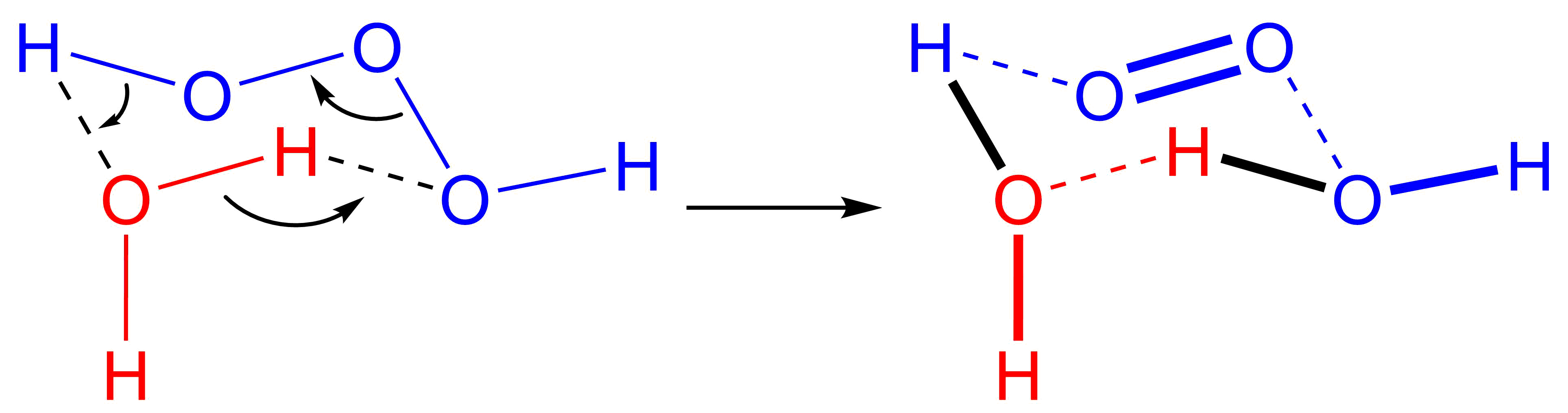
Trioxidane
Trioxidane (systematically named μ-trioxidanediidodihydrogen), also called dihydrogen trioxide, is an inorganic compound with the chemical formula (can be written as or ). It is one of the unstable hydrogen polyoxides. In aqueous solutions, trioxidane decomposes to form water and singlet oxygen: The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however, ozone is known to be generated from singlet oxygen, and the presumed mechanism is an antibody-catalyzed production of trioxidane from singlet oxygen. Preparation Trioxidane can be obtained in small, but detectable, amounts in reactions of ozone and hydrogen peroxide, or by the electrolysis of water. Larger quantities have been prepared by the reaction of ozone with organic reducing agents at low temperatures in a variety of organic solvents, such as the anthraquinone process. It is also formed during the decomposition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black soli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides. Ionic ozonides Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule. Inorganic ozonides are formed by burning potassium, rubidium, or caesium in ozone, or by treating the alkali metal hydroxide with ozone; this yields potassium ozonide, rubidium ozonide, and caesium ozonide respectively. They are very sensitive explosives that have to be handled at low temperatures in an atmosphere consisting of an inert gas. Lithium and sodium ozonide are extremely labile and must be prepared by low-temperature ion exchange starting from . Sodium ozonide, , which is prone to decomposition into NaOH and , was previously thought to be impossible to obtain in pure form. However, with the help of cryptands and methylamine, pure sodium ozonide may be obtained as red crystals isostructural to . Ionic ozonides are being investigate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen Heterocycles
Oxygen is the chemical element with the chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen Group (periodic table), group in the periodic table, a highly Chemical reaction, reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is Earth's Abundance of the chemical elements, most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element chemical bond, bind to form Allotropes of oxygen#Dioxygen, dioxygen, a colorless and odorless diatomic molecule, diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has Geological history of oxygen, changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |