|
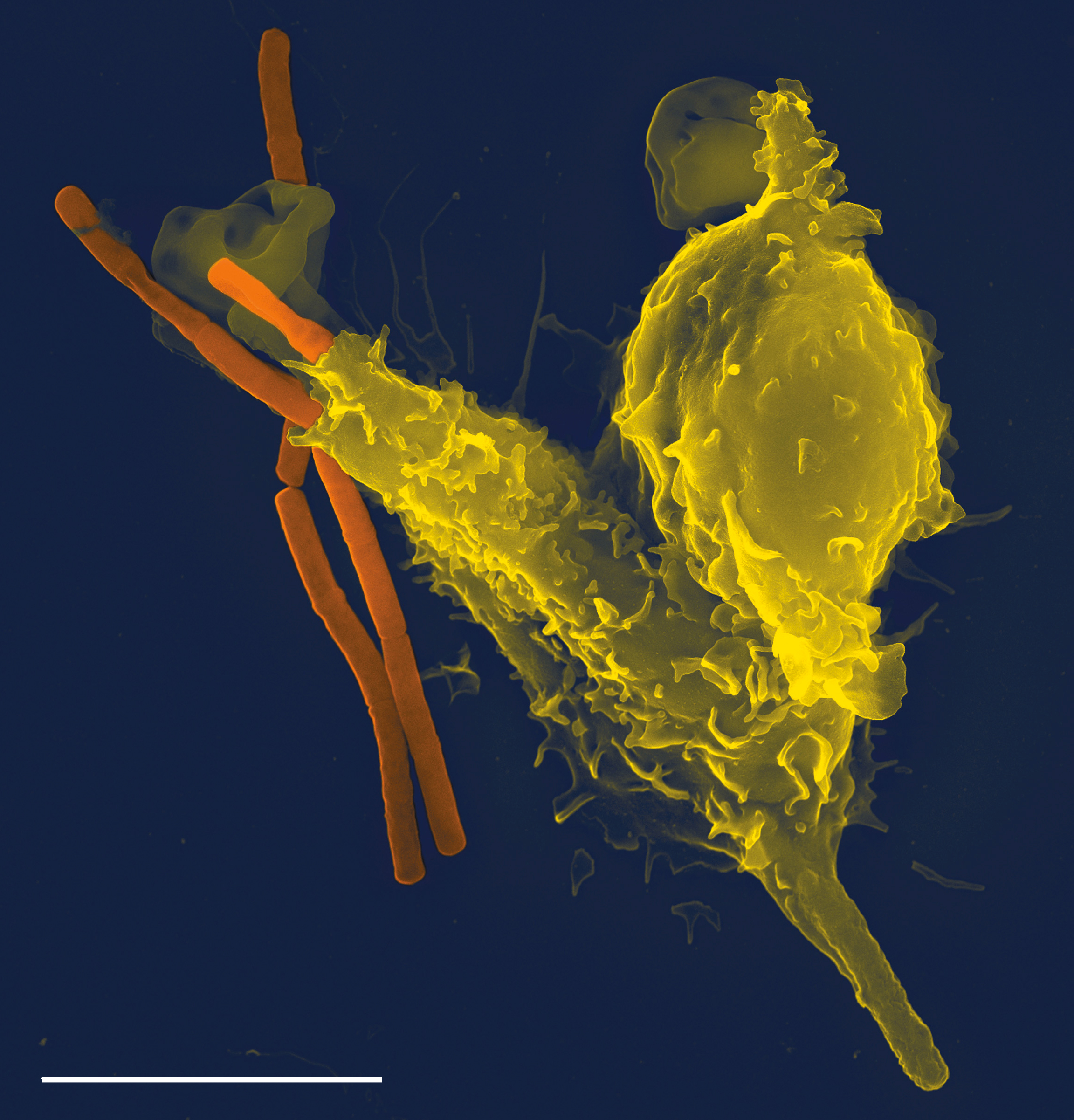
Mincle
Macrophage inducible Ca2+-dependent lectin receptor, (abbreviated to Mincle), is a member of the C-type lectin superfamily encoded by the gene CLEC4E. It is a pattern recognition receptor that can recognize glycolipids including mycobacterial cord factor, trehalose-6,6'-dimycolate (TDM). The mincle receptor binds a range of carbohydrate structures, predominantly containing glucose or mannose, and play an important role in recognition of bacterial glycolipids by the immune system. Upon activation by cord factor, Mincle binds the Fc receptor FcRγ and Syk. Cord factor also binds and activates the related C-type lectin MCL. Upon receptor stimulation is PKC-δ activated, which subsequently phosphorylates CARD9 that triggers recruitment of BCL10 and MALT1, leading to a CARD-CC/BCL10/MALT1 (CBM) signaling complex. This signaling complex in turn triggers downstream recruitment of TRAF6 and NF-κB activation. A wide range of ligands promote signalling through Mincle, including protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cord Factor
Cord factor, or trehalose dimycolate, is a glycolipid molecule found in the cell wall of '' Mycobacterium tuberculosis'' and similar species. It is the primary lipid found on the exterior of ''M. tuberculosis'' cells. Cord factor influences the arrangement of ''M. tuberculosis'' cells into long and slender formations, giving its name. Cord factor is virulent towards mammalian cells and critical for survival of ''M. tuberculosis'' in hosts, but not outside of hosts. Cord factor has been observed to influence immune responses, induce the formation of granulomas, and inhibit tumor growth. The antimycobacterial drug SQ109 is thought to inhibit TDM production levels and in this way disrupts its cell wall assembly. Structure A cord factor molecule is composed of a sugar molecule, trehalose (a disaccharide), composed of two glucose molecules linked together. Trehalose is esterified to two mycolic acid residues. One of the two mycolic acid residues is attached to the sixth carbon o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pattern Recognition Receptor
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines. The microbe-specific molecules that are recognized by a given PRR are called p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pattern Recognition Receptors
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines. The microbe-specific molecules that are recognized by a given PRR are called p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune System
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against virus infections. Other basic immune mechanisms evolved in ancient plants and animals and remain in their modern descendants. These mechanisms include phagocytosis, antimicrobial pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine-protein Kinase SYK
Tyrosine-protein kinase SYK, also known as spleen tyrosine kinase, is an enzyme which in humans is encoded by the ''SYK'' gene. Function SYK, along with ZAP70, is a member of the Syk family of tyrosine kinases. These cytoplasmic non-receptor tyrosine kinases share a characteristic dual SH2 domain separated by a linker domain. However, activation of SYK relies less on phosphorylation by Src family kinases than ZAP70. SYK and ZAP70 share a common evolutionary origin and split from a common ancestor in the jawed vertebrates. While Syk and ZAP70 are primarily expressed in hematopoietic tissues, a variety tissues express Syk. Within B and T cells, respectively, Syk and ZAP70 transmit signals from the B-cell receptor and T-cell receptor. Syk plays a similar role in transmitting signals from a variety of cell surface receptors including CD74, Fc receptor, and integrins. Function during development Mice that lack Syk completely (Syk−/−, Syk-knockout) die during embryonic deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PRKCD
Protein kinase C delta type (or PKC-δ) is an enzyme that in humans is encoded by the ''PRKCD'' gene. Function Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by the second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play distinct roles in cells. The protein encoded by this gene is one of the PKC family members. Studies both in human and mice demonstrate that this kinase is involved in B cell signaling and in the regulation of growth, apoptosis, and differentiation of a variety of cell types. Protein kinase C delta is also regulated by phosphorylation on various serine/threonine (e.g. T50, T141, S304, T451, T505, S506, T507, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD9
Caspase recruitment domain-containing protein 9 is an adaptor protein of the CARD-CC protein family, which in humans is encoded by the ''CARD9'' gene. It mediates signals from pattern recognition receptors to activate pro-inflammatory and anti-inflammatory cytokines, regulating inflammation and cell apoptosis. Homozygous mutations in CARD9 are associated with defective innate immunity against yeasts, like Candida and dermatophytes. Function CARD9 is a member of the CARD protein family, which is defined by the presence of a characteristic caspase-associated recruitment domain (CARD). CARD is a protein interaction domain known to participate in activation or suppression of CARD containing members of the caspase family, and thus plays an important regulatory role in cell apoptosis. This protein was identified by its selective association with the CARD domain of BCL10, a positive regulator of apoptosis and NF-κB activation. It is thought to function as a molecular scaffold for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BCL10
B-cell lymphoma/leukemia 10 is a protein that in humans is encoded by the ''BCL10'' gene. Like BCL2, BCL3, BCL5, BCL6, BCL7A, and BCL9, it has clinical significance in lymphoma. Function Bcl10 was identified by its translocation in a case of mucosa-associated lymphoid tissue (MALT) lymphoma. The protein encoded by this gene contains a caspase recruitment domain (CARD), and has been shown to induce apoptosis and to activate NF-κB. This protein is reported to interact with other CARD and coiled coil domain containing proteins including CARD9, -10, -11 and -14, which are thought to function as upstream regulators in NF-κB signaling. This protein is found to form a complex with the paracaspase MALT1, a protein encoded by another gene known to be translocated in MALT lymphoma. MALT1 and Bcl10 thought to synergize in the activation of NF-κB, and the deregulation of either of them may contribute to the same pathogenetic process that leads to the malignancy. Bcl10 is evolution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MALT1
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 is a protein that in humans is encoded by the ''MALT1'' gene. It's the human paracaspase. Function Genetic ablation of the paracaspase gene in mice and biochemical studies have shown that paracaspase is a crucial protein for T and B lymphocytes activation. It has an important role in the activation of the transcription factor NF-κB, in the production of interleukin-2 (IL-2) and in T and B lymphocytes proliferation Two alternatively spliced transcript variants encoding different isoforms have been described for this gene. In addition, a role for paracaspase has been shown in the innate immune response mediated by the zymosan receptor Dectin-1 in macrophages and dendritic cells, and in response to the stimulation of certain G protein-coupled receptors. Sequence analysis proposes that paracaspase has an N-terminal death domain, two central immunoglobulin-like domains involved in the binding to the B-cell lymphoma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD-CC Family
The CARD-CC protein family is defined by an evolutionary conserved CARD and a coiled-coil (CC) domain. Coiled-coils (CC) act as oligomerization domains for many proteins such as structural and motor proteins, and transcription factors. This means that monomers are converted to macromolecular complexes by polymerization. The protein family is ancient and can be found as far back as Cnidaria, but has almost exclusively been studied in humans and mice. Notably, the protein family is absent in insects and nematodes, which makes it impossible to study its function in the most popular invertebrate model organisms (Drosophila and C. elegans). In humans and other jawed vertebrates, the family consists of CARD9 and the three "CARD-containing MAGUK protein" (CARMA) proteins CARD11 (CARMA1), CARD14 (CARMA2) and CARD10 (CARMA3). Invertebrates only have a CARD9-like ancestral CARD-CC member, and the earliest occurrence of a CARD-CC member with the CARMA domain composition is in the jawless ver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |