|
Micelles
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloid, colloidal suspension (also known as associated colloidal system). A typical micelle in aqueous solution, water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the Lipid bilayer phase behavior, packing behavior of single-tail lipids in a lipid bilayer, bilayer. The difficulty filling all the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (water-in-oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micelle Scheme-en
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloid, colloidal suspension (also known as associated colloidal system). A typical micelle in aqueous solution, water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the Lipid bilayer phase behavior, packing behavior of single-tail lipids in a lipid bilayer, bilayer. The difficulty filling all the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (water-in-oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate (of detergents) is less likely than the polar carboxylate (of soap) to bind to calcium and other ions found in hard water. Definitions The word ''detergent'' is derived from the Latin adjective ''detergens'', from the verb ''detergere'', meaning to wipe or polish off. Detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to ''soap'' (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term ''detergent'' refers to household cleaning products such as ''laundry detergent'' or '' dish ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (biophysics)
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another (lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a set of rings. The ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Behaviour
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another (lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a set of rings. The ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer Phase Behavior
One property of a lipid bilayer is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a transition ( melt) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk, migrate over long distances.H. C. Berg, "Random Walks in Biology". Extended Paperback Ed. ed. 1993, Princeton, NJ: Princeton University Press. Motion constraints In contrast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (biophysics)
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another (lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a set of rings. The ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
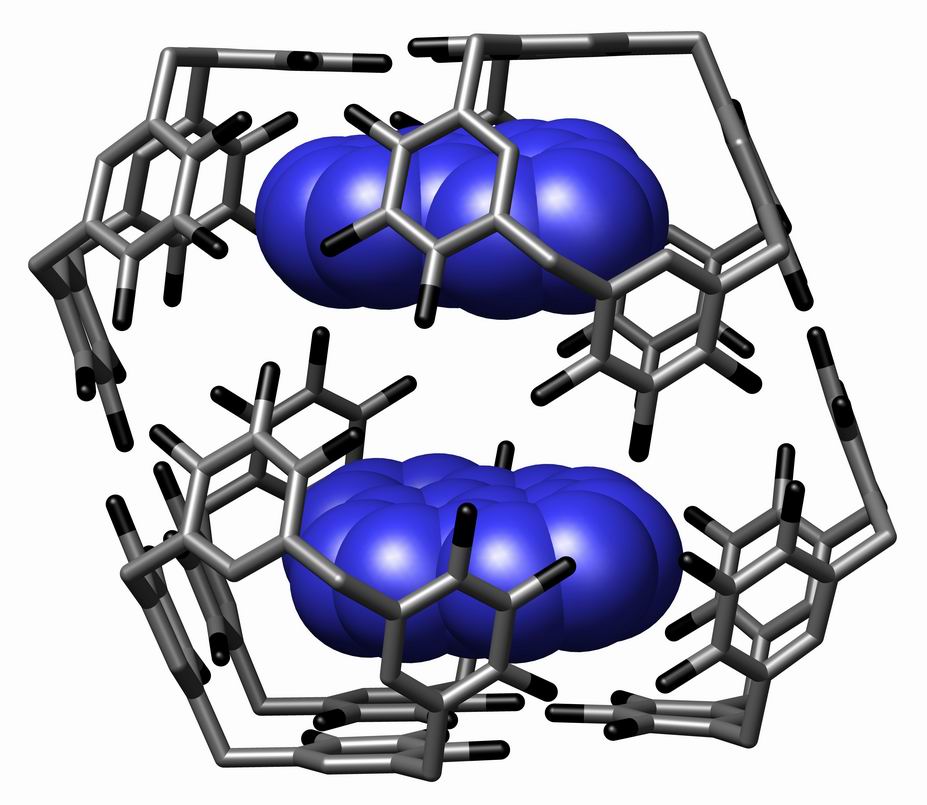
Supramolecular Assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies, and their realm of study is known as supramolecular chemistry. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions. The process by which a supramolecular assembly forms is called molecular self-assembly. Some try to dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphipathic
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compound is called amphiphilic or amphipathic. Common amphiphilic substances are soaps, detergents, and lipoproteins. The phospholipid amphiphiles are the major structural component of cell membranes. Amphiphiles are the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of the molecule are called bolaamphiphile, bolaamphiphilic. The micelles they form in the aggregate are prolate. Structure The lipophilic group is typically a large hydrocarbon Moiety (chemistry), moiety, such as a long chain of the form CH3(CH2)n, with n > 4. The hydrophilic group falls into one of the following categories: # charged groups #* anionic. Examples ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James William McBain
James William McBain FRS (March 22, 1882 – March 12, 1953) was a Canadian chemist. He gained a Master of Arts at Toronto University and a Doctor of Science at Heidelberg University. He carried out pioneering work in the area of micelles at the University of Bristol. As early as 1913 he postulated the existence of "colloidal ions", now known as micelles, to explain the good electrolytic conductivity of sodium palmitate solutions. He was elected a Fellow of the Royal Society in May 1923 He won their Davy Medal The Davy Medal is awarded by the Royal Society of London "for an outstandingly important recent discovery in any branch of chemistry". Named after Humphry Davy, the medal is awarded with a monetary gift, initially of £1000 (currently £2000). H ... in 1939. References Fellows of the Royal Society 1882 births 1953 deaths Canadian physical chemists {{Canada-scientist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps. Biological bilayers are usually composed of amphiphilic phosphol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |