|
MgO
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Peroxide
Magnesium peroxide (MgO2) is an odorless fine powder peroxide with a white to off-white color. It is similar to calcium peroxide because magnesium peroxide also releases oxygen by breaking down at a controlled rate with water. Commercially, magnesium peroxide often exists as a compound of magnesium peroxide and magnesium hydroxide. Structure O2, similarly to N2, has the ability to bind either side-on or end-on. The structure of MgO2 has been calculated as a triangular shape with the O2 molecule binding side-on to the magnesium. This arrangement is a result of the Mg+ donating charge to the oxygen and creating a Mg2+O22−. The bond between to O2 and the magnesium atom has an approximate dissociation energy of 90 kJ mol−1. In the solid state, MgO2 has a cubic pyrite-type crystal structure with 6-coordinate Mg2+ ions and O22− peroxide-groups, according to experimental data and evolutionary crystal structure prediction, the latter predicting a phase transition at the pressure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with lime (Ca(OH)2). A volume of (or 160,000 US gallons) of seawater gives about one ton of Mg(OH)2. Ca(OH)2 is far more soluble than Mg(OH)2 and drastically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the dissolution of : : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periclase
Periclase is a magnesium mineral that occurs naturally in Contact metamorphism, contact metamorphic rocks and is a major component of most basic refractory bricks. It is a Cubic crystal system, cubic form of magnesium oxide (magnesium, Mgoxygen, O). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite. It was first described in 1840 and named from the Greek περικλάω (to break around) in allusion to its cleavage. The Type locality (geology), type locality is Monte Somma, Somma-Vesuvius Complex, Naples Province, Campania, Italy. The old term for the mineral is ''magnesia''. Stones from the Magnesia on the Maeander, Magnesia region in ancient Anatolia contained both magnesium oxide and hydrated magnesium carbonate as well as iron oxides (such as magnetite). Thus these stones, called ''Stones from Magnesia'' in antiquity, with their unusual magnetic properties were the reason the terms ''magnet'' and ''m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium Oxide
Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste. Preparation and chemical properties Beryllium oxide can be prepared by calcining (roasting) beryllium carbonate, dehydrating beryllium hydroxide, or igniting metallic beryllium: :BeCO3 → BeO + CO2 :Be(OH)2 → BeO + H2O :2 Be + O2 → 2 BeO Igniting beryllium in air gives a mixture of BeO and the nitride Be3N2. Unlike the oxides formed by the other Group 2 elements (alkaline earth metals), beryllium oxide is amphoteric rather than basic. Berylliu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periclase
Periclase is a magnesium mineral that occurs naturally in Contact metamorphism, contact metamorphic rocks and is a major component of most basic refractory bricks. It is a Cubic crystal system, cubic form of magnesium oxide (magnesium, Mgoxygen, O). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite. It was first described in 1840 and named from the Greek περικλάω (to break around) in allusion to its cleavage. The Type locality (geology), type locality is Monte Somma, Somma-Vesuvius Complex, Naples Province, Campania, Italy. The old term for the mineral is ''magnesia''. Stones from the Magnesia on the Maeander, Magnesia region in ancient Anatolia contained both magnesium oxide and hydrated magnesium carbonate as well as iron oxides (such as magnetite). Thus these stones, called ''Stones from Magnesia'' in antiquity, with their unusual magnetic properties were the reason the terms ''magnet'' and ''m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Fume Fever
Metal fume fever, also known as brass founders' ague, brass shakes, zinc shakes, galvie flu, galvo poisoning, metal dust fever, welding shivers, or Monday morning fever, is an illness primarily caused by exposure to chemicals such as zinc oxide (ZnO), aluminium oxide (Al2O3), or magnesium oxide (MgO) which are produced as byproducts in the fumes that result when certain metals are heated. Other common sources are fuming silver, gold, platinum, chromium (from stainless steel), nickel, arsenic, manganese, beryllium, cadmium, cobalt, lead, selenium, and zinc. Welders are routinely exposed to the substances that cause metal fume fever from the base metal, plating, or filler. The most common form of exposure among welders occurs when welding galvanized steel, of which zinc is the primary component of the galvanization process. Galvanized metal must be thoroughly cleaned using an angle grinder or other abrasive means to remove the galvanized coating before welding or burning. Brazing and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
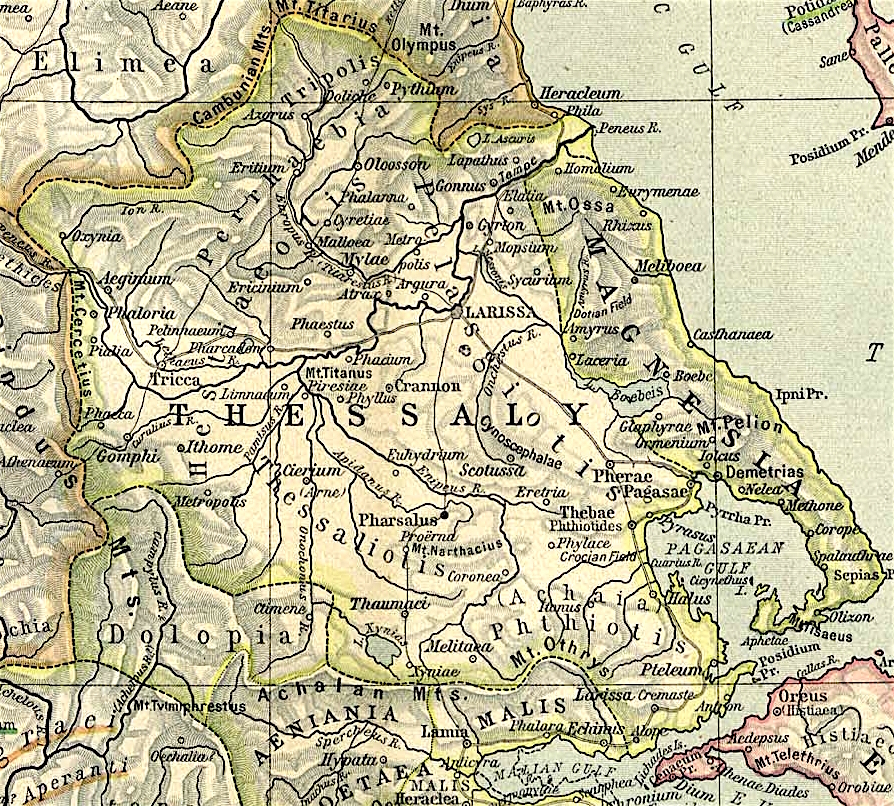
Ancient Magnesia
Anciently, Magnesia ( grc, Μαγνησία) was a region of Ancient Greece, eventually absorbed by ancient Thessaly. Originally inhabited by the Magnetes (Μάγνητες), Magnesia was the long and narrow slip of country between Mounts Ossa and Pelion on the west and the sea on the east, and extending from the mouth of the Peneius on the north to the Pagasaean Gulf on the south. The Magnetes were members of the Amphictyonic League, and were settled in this district in the Homeric times, and mentioned in the ''Iliad''. The Thessalian Magnetes are said to have founded the Asiatic cities of Magnesia ad Sipylum and Magnesia on the Maeander.Aristot. ''ap. Athen.'' 4.173; Conon 29; The towns of Magnesia were: Aesonis, Aphetae, Boebe, Casthanaea, Cercinium, Coracae, Demetrias, Eurymenae, Glaphyrae, Homole or Homolium, Iolcus, Magnesia, Meliboea, Methone, Mylae, Nelia, Olizon, Pagasae, Rhizus, Spalaethra, and Thaumacia Thaumacia or Thaumakie ( grc, Θαυμακία or Θαυ� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesia Negra
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laboratory in the form of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic charge. Atoms that gain electrons make negatively charged ions (called anions). Atoms that lose electrons make positively charged ions (called cations). This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like or . In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms. It is important to recognize that ''clean'' ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |