|
Metal Carbon Dioxide Complex
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels. Structural trends : Carbon dioxide binds to metals in only a few ways. The bonding mode depends on the electrophilicity and basicity of the metal centre. Most common is the η2-CO2 coordination mode as illustrated by Aresta's complex, Ni(CO2)(tricyclohexylphosphine, PCy3)2, which was the first reported complex of CO2. This square-planar compound is a derivative of Ni(II) with a reduced CO2 ligand. In rare cases, CO2 binds to metals as a Lewis base through its oxygen centres, but such adducts are weak and mainly of theoretical interest. A varie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terminal Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–hydrogen Bond Activation
In organic chemistry, carbon–hydrogen bond functionalization ( functionalization) is a type of organic reaction in which a carbon–hydrogen bond is cleaved and replaced with a bond (where X is usually carbon, oxygen, or nitrogen). The term usually implies that a transition metal is involved in the cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "" intermediate. The intermediate of this first step (known as activation and sometimes used interchangeably with functionalization) can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scheme 4 Pincer Pd Complexes For Catalytic Carboxylation
A scheme is a systematic plan for the implementation of a certain idea. Scheme or schemer may refer to: Arts and entertainment * ''The Scheme'' (TV series), a BBC Scotland documentary series * The Scheme (band), an English pop band * ''The Scheme'', an action role-playing video game for the PC-8801, made by Quest Corporation * Schemer (comics), Richard Fisk, a Marvel Comics villain turned antihero * Horace Schemer, a fictional character in the TV series ''Shining Time Station'' * Schemee, a fictional child character and Schemer's nephew in the TV Series ''Shining Time Station'' * ''Schemers'' (film), a Scottish film Other uses * Classification scheme, eg a thesaurus, a taxonomy, a data model, or an ontology * Scheme (programming language), a minimalist dialect of Lisp * Scheme (mathematics), a concept in algebraic geometry * Scheme (linguistics), a figure of speech that changes a sentence's structure * Scam, an attempt to swindle or cheat people through deception **Get-rich-quick ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
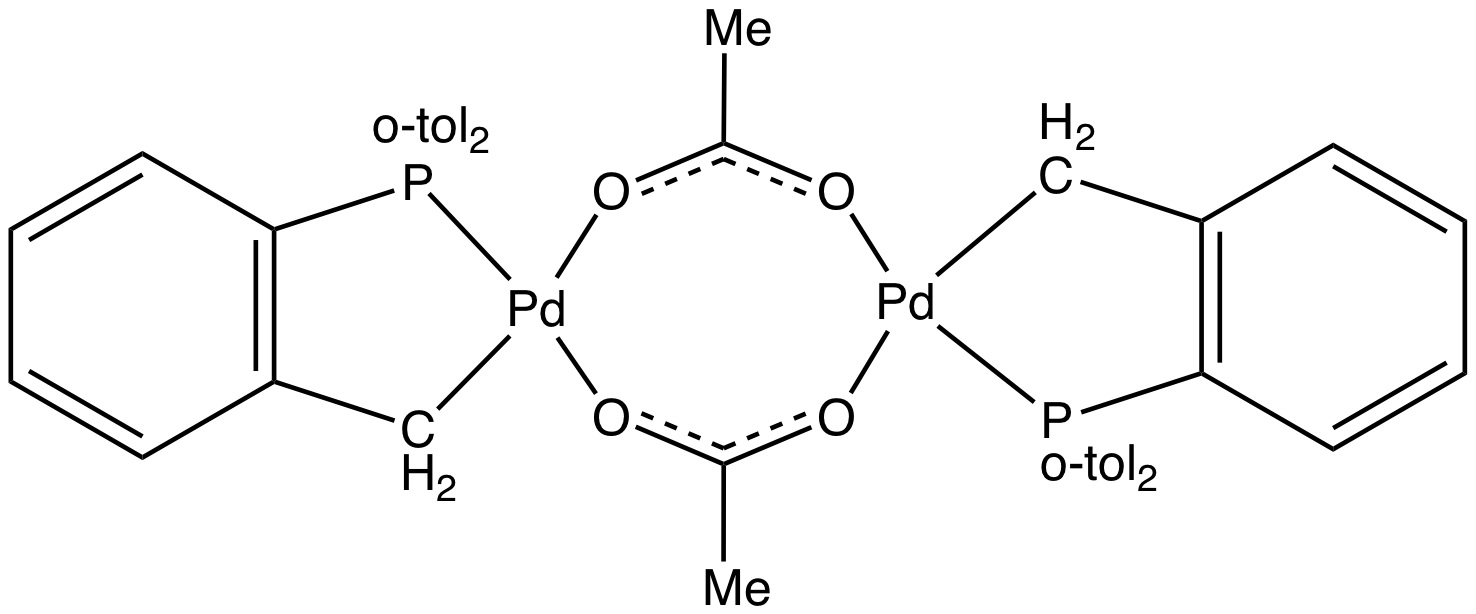
Palladium(II) Acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions. Structure With a 1:2 stoichiometric ratio of palladium atoms and acetate ligands, the compound exists as molecular and polymeric forms with the trimeric form being the dominant form in the solid state and in solution. Pd achieves approximate square planar coordination in both forms. As prepared by Geoffrey Wilkinson and coworkers in 1965 and later characterized by Skapski and Smart in 1970 by single crystal X-ray diffraction, palladium(II) acetate is a red-brown solid that crystallizes as monoclinic plates. It has a trimeric structure, consisting of an equilateral triangle of Pd atoms each pair of which is bridged with two acetate groups in a butterfly conformat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium. Structure and bonding The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). In Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlMe3. Synthesis TMA is prepared via a two-step process that can be summarized as follows: :2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl Applications Catalysis Starting with the invention of Ziegler-Natta catalysis, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoaluminium Chemistry
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins. History The first organoaluminium compound (C2H5)3Al2I3 was discovered in 1859. Organoaluminium compounds were, however, little known until the 1950s when Karl Ziegler and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic olefin polymerization. This line of research ultimately resulted in the Nobel Prize to Ziegler. Structure and bonding Aluminium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organozinc Compound
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compounds' (Patai Series, (Eds. Z. Rappoport and I. Marek), John Wiley & Sons: Chichester, UK, 2006, .''Organozinc reagents – A Practical Approach'', (Eds. P. Knochel and P. Jones), Oxford Medical Publications, Oxford, 1999, . Organozinc compounds were among the first organometallic compounds made. They are less reactive than many other analogous organometallic reagents, such as Grignard and organolithium reagents. In 1848 Edward Frankland prepared the first organozinc compound, diethylzinc, by heating ethyl iodide in the presence of zinc metal.E. Frankland, Liebigs Ann. Chem.,1849, 71, 171 This reaction produced a volatile colorless liquid that spontaneous combusted upon contact with air. Due to their pyrophoric nature, organozinc compounds a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as diene#Classes, cumulated dienes. The parent compound of this class is propadiene, which is itself also called ''allene''. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with bonding. History For many years, allenes were viewed as curiosities but thought to be synthetically useless and difficult to prepare and to work with.The Chemistry of the Allenes (vol. 1−3); Landor, S. R., Ed.; cademic Press: London, 1982. Reportedly, the first synthesis of an allene, glutinic acid, was performed in an attempt to prove the non-existence of this class of compounds. The situation began to change in the 1950s, and more than 300 papers on allenes have been published in 2012 alone. These compounds are not just interesting intermediates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scheme 3 Copper Catalysed Boracarboxylation Of Internal Alkynes
A scheme is a systematic plan for the implementation of a certain idea. Scheme or schemer may refer to: Arts and entertainment * ''The Scheme'' (TV series), a BBC Scotland documentary series * The Scheme (band), an English pop band * ''The Scheme'', an action role-playing video game for the PC-8801, made by Quest Corporation * Schemer (comics), Richard Fisk, a Marvel Comics villain turned antihero * Horace Schemer, a fictional character in the TV series ''Shining Time Station'' * Schemee, a fictional child character and Schemer's nephew in the TV Series ''Shining Time Station'' * ''Schemers'' (film), a Scottish film Other uses * Classification scheme, eg a thesaurus, a taxonomy, a data model, or an ontology * Scheme (programming language), a minimalist dialect of Lisp * Scheme (mathematics), a concept in algebraic geometry * Scheme (linguistics), a figure of speech that changes a sentence's structure * Scam, an attempt to swindle or cheat people through deception **Get-rich-quick ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |