Metal carbon dioxide complex on:
[Wikipedia]
[Google]
[Amazon]
Metal carbon dioxide complexes are coordination complexes that contain
 Carbon dioxide binds to metals in only a few ways. The bonding mode depends on the electrophilicity and basicity of the metal centre. Most common is the η2-CO2 coordination mode as illustrated by Aresta's complex, Ni(CO2)( PCy3)2, which was the first reported complex of CO2. This square-planar compound is a derivative of Ni(II) with a reduced CO2 ligand. In rare cases, CO2 binds to metals as a
Carbon dioxide binds to metals in only a few ways. The bonding mode depends on the electrophilicity and basicity of the metal centre. Most common is the η2-CO2 coordination mode as illustrated by Aresta's complex, Ni(CO2)( PCy3)2, which was the first reported complex of CO2. This square-planar compound is a derivative of Ni(II) with a reduced CO2 ligand. In rare cases, CO2 binds to metals as a
 Apart from transmetallation, there are other approaches forming Cu-C bond. C-H functionalization is a straightforward and atom economic method. Base can help deprotonate acidic C-H protons and form Cu-C bond. Phenanthroline)Cu(PR3).html" ;"title="Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)">Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)catalyst effect C-H carboxylation on terminal alkynes together with Cs2CO3. NHC-Cu-H species to deprotonate acidic proton to effect carboxylation of terminal alkynes. Cu-H species were generated from Cu-F and organosilanes. The carboxylate product was trapped by silyl fluoride to get silyl ether. For non-acidic C-H bonds, directed metalation with iBu3Al(TMP)Li is adopted followed by transmetallation with copper to get Cu-C bond.
Apart from transmetallation, there are other approaches forming Cu-C bond. C-H functionalization is a straightforward and atom economic method. Base can help deprotonate acidic C-H protons and form Cu-C bond. Phenanthroline)Cu(PR3).html" ;"title="Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)">Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)catalyst effect C-H carboxylation on terminal alkynes together with Cs2CO3. NHC-Cu-H species to deprotonate acidic proton to effect carboxylation of terminal alkynes. Cu-H species were generated from Cu-F and organosilanes. The carboxylate product was trapped by silyl fluoride to get silyl ether. For non-acidic C-H bonds, directed metalation with iBu3Al(TMP)Li is adopted followed by transmetallation with copper to get Cu-C bond.  Carbometallation to alkynes and
Carbometallation to alkynes and
 Palladium has shown huge power to catalyze C-H functionalization. If the Pd-C intermediate in carboxylation reaction comes from C-H activation, such methodology must promote metal catalyzed carboxylation to a much higher level in utility. Iwasawa and co-workers reported direct carboxylation by styrenyl C-H activation generating coumarin derivatives. Benzene rings with different electronic properties and some
Palladium has shown huge power to catalyze C-H functionalization. If the Pd-C intermediate in carboxylation reaction comes from C-H activation, such methodology must promote metal catalyzed carboxylation to a much higher level in utility. Iwasawa and co-workers reported direct carboxylation by styrenyl C-H activation generating coumarin derivatives. Benzene rings with different electronic properties and some


carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s. Aside from the fundamental interest in the coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.
Structural trends
: Carbon dioxide binds to metals in only a few ways. The bonding mode depends on the electrophilicity and basicity of the metal centre. Most common is the η2-CO2 coordination mode as illustrated by Aresta's complex, Ni(CO2)( PCy3)2, which was the first reported complex of CO2. This square-planar compound is a derivative of Ni(II) with a reduced CO2 ligand. In rare cases, CO2 binds to metals as a
Carbon dioxide binds to metals in only a few ways. The bonding mode depends on the electrophilicity and basicity of the metal centre. Most common is the η2-CO2 coordination mode as illustrated by Aresta's complex, Ni(CO2)( PCy3)2, which was the first reported complex of CO2. This square-planar compound is a derivative of Ni(II) with a reduced CO2 ligand. In rare cases, CO2 binds to metals as a Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
through its oxygen centres, but such adducts are weak and mainly of theoretical interest. A variety of multinuclear complexes are also known often involving Lewis basic and Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
ic metals, e.g. metallacarboxylate salts (C5H5)Fe(CO)2CO2−K+. In multinuclear cases (compounds containing more than one metal), more complicated and more varied coordination geometries are observed. One example is the unsymmetrical compound containing four rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
centres, CO)5ReCO2Re(CO)4sub>2. Carbon dioxide can also bind to ligands on a metal complex (vs just the metal), e.g. by converting hydroxy ligands to carbonato ligands.
Reactions
Transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
carbon dioxide complexes undergo a variety of reactions. Metallacarboxylic acids protonate at oxygen and eventually convert to metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe ch ...
complexes:
: nMCO2sup>− + 2 H+ → nMCOsup>+ + H2O
This reaction is relevant to the potential catalytic conversion of CO2 to fuels.
Carbonation of metal-carbon bonds
Insertion into Cu-C bonds
N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
(NHC) supported CuI complexes catalyze carboxylation of organoboronic esters. The catalyst forms ''in situ'' from CuCl, an NHC ligand, and KOtBu. Copper ''tert''-butoxide can transmetallate with the organoboronic ester to generate the CuI-C bond, which intermediate can insert into CO2 smoothly to get the respective carboxylate. Salt metathesis with KOtBu releases product and regenerates catalyst (Scheme 2).
 Apart from transmetallation, there are other approaches forming Cu-C bond. C-H functionalization is a straightforward and atom economic method. Base can help deprotonate acidic C-H protons and form Cu-C bond. Phenanthroline)Cu(PR3).html" ;"title="Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)">Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)catalyst effect C-H carboxylation on terminal alkynes together with Cs2CO3. NHC-Cu-H species to deprotonate acidic proton to effect carboxylation of terminal alkynes. Cu-H species were generated from Cu-F and organosilanes. The carboxylate product was trapped by silyl fluoride to get silyl ether. For non-acidic C-H bonds, directed metalation with iBu3Al(TMP)Li is adopted followed by transmetallation with copper to get Cu-C bond.
Apart from transmetallation, there are other approaches forming Cu-C bond. C-H functionalization is a straightforward and atom economic method. Base can help deprotonate acidic C-H protons and form Cu-C bond. Phenanthroline)Cu(PR3).html" ;"title="Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)">Phenanthroline.html" ;"title="Phenanthroline">Phenanthroline)Cu(PR3)catalyst effect C-H carboxylation on terminal alkynes together with Cs2CO3. NHC-Cu-H species to deprotonate acidic proton to effect carboxylation of terminal alkynes. Cu-H species were generated from Cu-F and organosilanes. The carboxylate product was trapped by silyl fluoride to get silyl ether. For non-acidic C-H bonds, directed metalation with iBu3Al(TMP)Li is adopted followed by transmetallation with copper to get Cu-C bond. Allylic
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . ...
C-H bonds and phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
C-H bonds got carboxylated with this approach by Hou and co-workers:
 Carbometallation to alkynes and
Carbometallation to alkynes and allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as diene#Classes, cumulated dienes. The parent compound of this class is propa ...
s using organozinc
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compoun ...
and organoaluminum
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monome ...
reagents followed by transmetallation to copper is also a strategy to initiate carboxylation. Trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industriall ...
is able to insert into unbiased aliphatic internal alkynes with syn fashion directed by ether directing group. Vinyl copper complexes are formed by transmetallation and carboxylation is realized with a similar pathway giving tetrasubstituted aliphatic vinyl carboxylic acids. In this case, regioslectivity is controlled by the favor of six-membered aluminum ring formation. Furthermore, carboxylation can be achieved on ynamides and allenamides using less reactive dimethyl zinc via similar approach.
Insertion in Pd-C bonds
In the presence ofpalladium acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in man ...
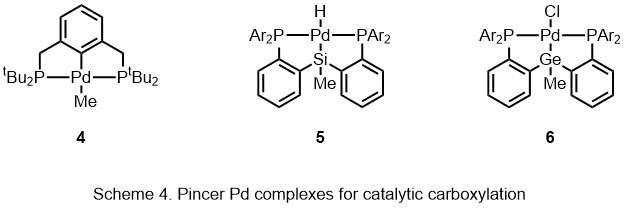
under 1-30 bar of CO2, simple aromatic compounds convert to aromatic carboxylic acids. A PSiP-pincer ligand (5) promotes carboxylation of allene without using pre-functionalized substrates. Catalyst regeneration, Et3Al was added to do transmetallation with palladium. Catalyst is regenerated by the following β-H elimination. Apart from terminal allenes, some of internal allenes are also tolerated in this reaction, generating allyl carboxylic acid with the yield between 54% and 95%. This system was also applied to 1,3-diene, generating carboxylic acid in 1,2 addition fashion. In 2015, Iwasawa ''et al.'' reported the germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
analogue (6) and combined CO2 source together with hydride source to formate salts.
 Palladium has shown huge power to catalyze C-H functionalization. If the Pd-C intermediate in carboxylation reaction comes from C-H activation, such methodology must promote metal catalyzed carboxylation to a much higher level in utility. Iwasawa and co-workers reported direct carboxylation by styrenyl C-H activation generating coumarin derivatives. Benzene rings with different electronic properties and some
Palladium has shown huge power to catalyze C-H functionalization. If the Pd-C intermediate in carboxylation reaction comes from C-H activation, such methodology must promote metal catalyzed carboxylation to a much higher level in utility. Iwasawa and co-workers reported direct carboxylation by styrenyl C-H activation generating coumarin derivatives. Benzene rings with different electronic properties and some heteroaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
rings are tolerated in this reaction with yield from 50% to 90%. C-H activation was demonstrated by crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
study.
Insertion by Rh-C bonds
Similar to Cu(I) chemistry mentioned above, Rh(I) complexes can also transmetallate with arylboronic esters to get aryl rhodium intermediates, to which CO2 is inserted giving carboxylic acids. Later, Iwasawa ''et al''. described C-H carboxylation strategy. Rh(I) undergoes oxidative addition to aryl C-H bond followed by transmetallation with alkyl aluminum species. Ar-Rh(I) regenerates by reductive elimination releasing methane. Ar-Rh(I) attacks CO2 then transmetallates with aryl boronic acid to release the boronic acid of product, giving final carboxylic acid by hydrolysis. Directed and non-directed versions are both achieved. Iwasawa and co-workers developed Rh(I) catalyzed carbonation reaction initiated by Rh-H insertion to vinylarenes. In order to regenerate reactive Rh-H after nucleophilic addition to CO2,photocatalytic
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the abi ...
proton-coupled electron transfer A Proton-coupled electron transfer (PCET) is a chemical reaction that involves the transfer of electrons and protons from one atom to another. The term was originally coined for single proton, single electron processes that are concerted, but the ...
approach was adopted. In this system, excess amount of diethylpropylethylamine works as sacrificial electron donor (Scheme 5).

Insertion by Ni-C bond
Carboxylation of benzyl halides has been reported. The reaction mechanism is proposed to involve oxidative addition of benzyl chloride to Ni(0). The Ni(II) benzyl complex is reduced to Ni(I), e.g., by zinc, which inserts CO2 delivering the nickel carboxylate. Reduction of the Ni(I) carboxylate to Ni(0) releases the zinc carboxylate (Scheme 6). Similarly, such carboxylation has been achieved on aryl and benzyl pivalate, alkyl halides, and allyl esters.
References
{{Coordination complexes Coordination complexes Inorganic chemistry Organometallic chemistry Transition metals