|
Menthol (data Page)
This page provides supplementary chemical data on Menthol. Material Safety Data Sheet The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely ...) for this chemical from a reliable source such aSIRIor the links below, and follow its directions.(''l''-form)(DL or racemic form)(''l''-form)Menthol Eucalyptus ointment Structure and properties Thermodynamic properties Spectral data References {{reflist Chemical data pages Chemical data pages cleanup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Menthol
Menthol is an organic compound, more specifically a monoterpenoid, made synthetically or obtained from the oils of corn mint, peppermint, or other mints. It is a waxy, clear or white crystalline substance, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1''R'',2''S'',5''R'') configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation. Menthol also acts as a weak κ-opioid receptor agonist. Structure Natural menthol exists as one pure stereoisomer, nearly always the (1''R'',2''S'',5''R'') form (bottom left corner of the diagram below). The eight possible stereoisomers are: : In the natural compound, the isopropyl group is in the ''trans'' orientation to both the methyl and hydroxyl groups. Thus, it can be drawn in any of the ways shown: : The (+)- and (−)-enantiomers of menthol are the most stable among ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Entropy Change Of Vaporization
In thermodynamics, the entropy of vaporization is the increase in entropy upon vaporization of a liquid. This is always positive, since the degree of disorder increases in the transition from a liquid in a relatively small volume to a vapor or gas occupying a much larger space. At standard pressure , the value is denoted as and normally expressed in joules per mole-kelvin, J/(mol·K). For a phase transition such as vaporization or fusion (melting), both phases may coexist in equilibrium at constant temperature and pressure, in which case the difference in Gibbs free energy is equal to zero: : \Delta G_\text = \Delta H_\text - T_\text \times \Delta S_\text = 0, where \Delta H_\text is the heat or enthalpy of vaporization. Since this is a thermodynamic equation, the symbol refers to the absolute thermodynamic temperature, measured in kelvins (K). The entropy of vaporization is then equal to the heat of vaporization divided by the boiling point: : \Delta S_\text = \frac. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13 NMR
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR ( NMR) and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the isotope. The main carbon isotope, is not detected. Although much less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds. Chemical shifts 13C NMR chemical shifts follow the same principles as those of 1H, although the typical range of chemical shifts is much larger than for 1H (by a factor of about 20). The chemical shift reference standard for 13C is the carbons in tetramethylsilane (TMS), whose chemical shift is considered to be 0.0 ppm. ImageSize = width:540 height:440 AlignBar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H (hydrogen-1; i.e. having a proton for a nucleus). Simple NMR spectra are recorded in solution, and solvent protons must not be allowed to interfere. Deuterated (deuterium = 2H, often symbolized as D) solvents especially for use in NMR are preferred, e.g. deuterated water, D2O, deuterated acetone, (CD3)2CO, deuterated methanol, CD3OD, deuterated dimethyl sulfoxide, (CD3)2SO, and deuterated chloroform, CDCl3. However, a solvent without hydrogen, such as carbon tetrachloride, CCl4 or carbon disulfide, CS2, may also be used. Historically, deuterated solvents were supplied with a small amount (typically 0.1%) of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMR Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. The principle of NMR usually involves three sequential steps: # The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. # The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Absorptivity
Molar may refer to: *Molar (tooth), a kind of tooth found in mammals *Molar (grape), another name for the Spanish wine grape Listan Negro *Molar (unit), a unit of concentration equal to 1 mole per litre *Molar mass *Molar volume *El Molar, Tarragona, a village in the comarca (county) of Priorat, province of Tarragona in the autonomous region of Catalonia, Spain *El Molar, Madrid, a town in the north of the Community of Madrid in the road to Burgos, after San Agustín de Guadalix See also * Moler Moler (previously called Snuff) are a power pop band which formed in 1993 as a three-piece with founding mainstays Helen Cattanach on bass guitar and lead vocals and Julien Poulsen on lead guitar. They featured a changing line-up of drummers and ..., a power-pop band from Australia * Moler (surname) {{disambig, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, -er, American spelling) is a units of measurement, unit of length in the International System of Units (SI), equal to one billionth (short scale) of a metre () and to 1000 picometres. One nanometre can be expressed in scientific notation as , and as metres. History The nanometre was formerly known as the millimicrometre – or, more commonly, the millimicron for short – since it is of a micron (micrometre), and was often denoted by the symbol mμ or (more rarely and confusingly, since it logically should refer to a ''millionth'' of a micron) as μμ. Etymology The name combines the SI prefix ''nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
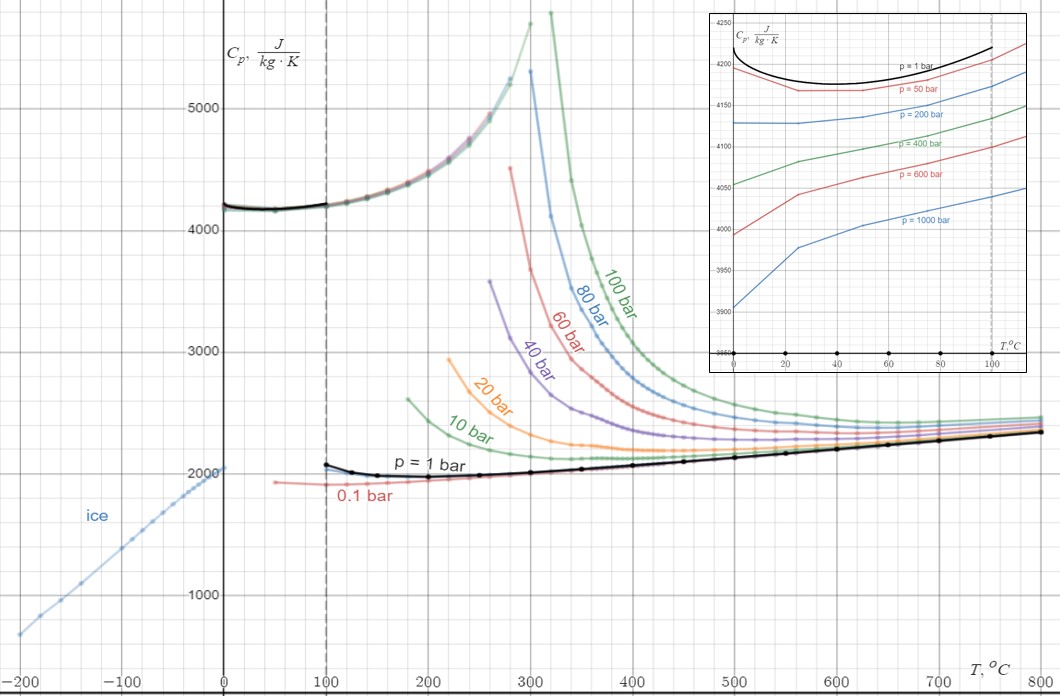
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)