|
Menshutkin Reaction
In organic chemistry, the Menshutkin reaction converts a tertiary amine into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides. The reaction is the method of choice for the preparation quaternary ammonium salts. Some phase transfer catalysts (PTC) can be prepared according to the Menshutkin reaction, for instance the synthesis of triethyl benzyl ammonium chloride (TEBA) from triethylamine and benzyl chloride: Scope Reactions are typically conducted in polar solvents such as alcohols. Alkyl iodides are superior alkylating agents relative to the bromides, which in turn are superior to chlorides. As is typical for an SN2 process, benzylic, allylic, and α-carbonylated alkyl halides are excellent reactants. Even though alkyl chlorides are poor alkylating agents (''gem''-dichlorides especially so), amines should not be handled in chlorinated solvents such as dichloromethane and dichlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nikolai Menshutkin
Nikolai Aleksandrovich Menshutkin (russian: Николай Александрович Меншуткин; – ) was a Russian chemist who discovered the process of converting a tertiary amine to a quaternary ammonium salt via the reaction with an alkyl halide, now known as the Menshutkin reaction. Biography Menshutkin was born in a merchant family as the sixth son of Alexander Nikolaevitch Menshutkin. He graduated with honors from gymnasium in December 1857, but only by autumn 1858 managed to enroll to the Saint Petersburg State University, as he was still under the prescribed age of 16. He studied at the faculty of physics and mathematics and was nearly expelled in the autumn of 1861 due to some political disturbances. Nevertheless, by the spring of 1862 he attained the master's degree. During the last years he became interested in chemistry, which he studied under Dmitri Mendeleev. While he acquired a sufficient knowledge of theory he was lacking practice, as at that time the en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with p''K''a of the conjugate acid of 11.0.{{cite journal , title=Azatriquinanes: Synthesis, Structure, and Reactivity , author1=Hext, N. M. , author2=Hansen, J. , author3=Blake, A. J. , author4=Hibbs, D. E. , author5=Hursthouse, M. B. , author6=Shishkin, O. V. , author7=Mascal, M. , journal=J. Org. Chem. , year=1998 , volume=63 , issue=17 , pages=6016–6020 , doi=10.1021/jo980788s , pmid=11672206 It can be prepared by reduction of quinuclidone. In alkane solvents quinuclidine is a Lewis base that forms adducts with a variety of Lewis acids. The compound is structurally related to DABCO, in which the other bridgehead is also nitrogen, and to tropane, which has a slightly different carbon frame. Quinuclidine is found as a structural component of some biomolecules including quinine Quinine is a medication used to treat malaria and babesiosis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocylization. Pioneering work was reported for studies on terpenoid macrocycles. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Some macrocyclizations are favored using template reactions. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size. The crown ethers are often generated in the presence of an alkali metal cation, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selectfluor
Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. It is a colourless salt that tolerates air and even water. It has been commercialized for use for electrophilic fluorination. Preparation Selectfluor is synthesized by the ''N''-alkylation of diazabicyclo .2.2ctane (DABCO) with dichloromethane, followed by ion exchange with sodium tetrafluoroborate (replacing the chloride counterion for the tetrafluoroborate). The resulting salt is treated with elemental fluorine and sodium tetrafluoroborate: : Mechanism of fluorination Electrophilic fluorinating reagents could in principle operate by electron transfer pathways or an SN2 attack at fluorine. This distinction has not been decided. By using a charge-spin separated probe, it was possible to show that the electrophilic fluorination of stilbenes with Selectfluor proceeds through an SET/fluorine atom tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
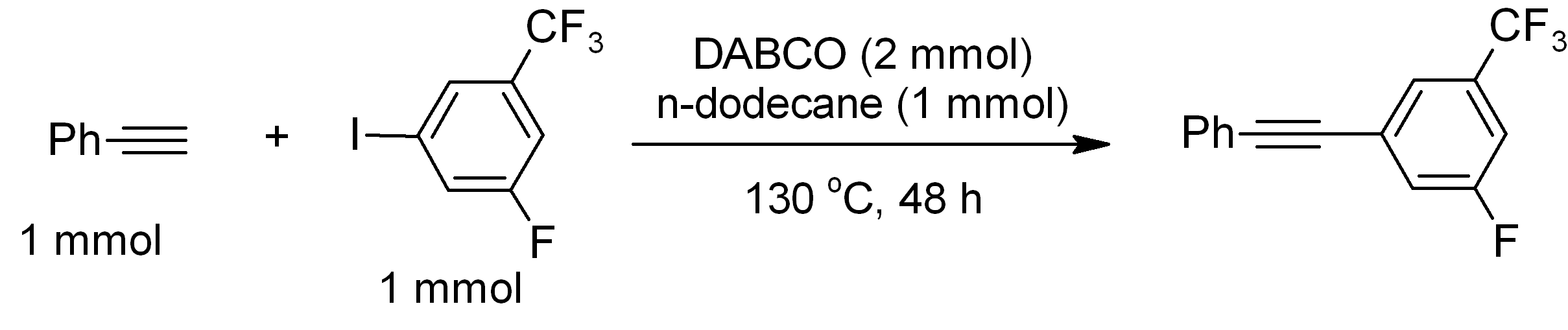
DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : Lewis ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block. Preparation Benzyl chloride is prepared industrially by the gas-phase photochemical reaction of toluene with chlorine: :C6H5CH3 + Cl2 → C6H5CH2Cl + HCl In this way, approximately 100,000 tonnes are produced annually. The reaction proceeds by the free radical process, involving the intermediacy of free chlorine atoms. Side products of the reaction include benzal chloride and benzotrichloride. Other methods of production exist, such as the Blanc chloromethylation of benzene. Benzyl chloride was first prepared from treatment of benzyl alcohol with hydrochloric acid. Uses and reactions Industrially, benzyl chloride is the precursor to benzyl esters, which are used as plasticizers, flavorants, and perfumes. Phenylacetic acid, a precursor to pharmaceuticals, is produced from benzyl cyan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The |
Phase Transfer Catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst. By using a PTC process, one can achieve faster reactions, obtain higher conversions or yields, make fewer byproducts, eliminate the need for expensive or dangerous solvents that will dissolve all the reactants in one phase, eliminate the need for expensive raw materials and/or minimize waste problems. Phase-transfer catalysts are especially useful in green chemistry—by allowing the use of water, the nee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |