|
Maxwell's Relations
file:Thermodynamic map.svg, 400px, Flow chart showing the paths between the Maxwell relations. P is pressure, T temperature, V volume, S entropy, \alpha coefficient of thermal expansion, \kappa compressibility, C_V heat capacity at constant volume, C_P heat capacity at constant pressure. Maxwell's relations are a set of equations in thermodynamics which are derivable from the symmetry of second derivatives and from the definitions of the thermodynamic potentials. These relations are named for the nineteenth-century physicist James Clerk Maxwell. Equations The structure of Maxwell relations is a statement of equality among the second derivatives for continuous functions. It follows directly from the fact that the order of differentiation of an analytic function of two variables is irrelevant ( Schwarz theorem). In the case of Maxwell relations the function considered is a thermodynamic potential and x_i and x_j are two different natural variables for that potential, we ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equations
Thermodynamics is expressed by a mathematical framework of ''thermodynamic equations'' which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics. Introduction One of the fundamental thermodynamic equations is the description of thermodynamic work in analogy to mechanical work, or weight lifted through an elevation against gravity, as defined in 1824 by French physicist Sadi Carnot. Carnot used the phrase motive power for work. In the footnotes to his famous ''On the Motive Power of Fire'', he states: “We use here the expression ''motive power'' to express the useful effect that a motor is capable of producing. This effect can always be likened to the elevation of a weight to a certain height. It has, as we know, as a measure, the product of the weight multiplied by the height to which it is raised.” With the inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grand Potential
The grand potential is a quantity used in statistical mechanics, especially for irreversible processes in open systems. The grand potential is the characteristic state function for the grand canonical ensemble. Definition Grand potential is defined by : \Phi_ \ \stackrel\ U - T S - \mu N where ''U'' is the internal energy, ''T'' is the temperature of the system, ''S'' is the entropy, μ is the chemical potential, and ''N'' is the number of particles in the system. The change in the grand potential is given by : \begin d\Phi_ & = dU - TdS - SdT - \mu dN - Nd\mu \\ & = - P dV - S dT - N d\mu \end where ''P'' is pressure and ''V'' is volume, using the fundamental thermodynamic relation (combined first and second thermodynamic laws); :dU = TdS - PdV + \mu dN When the system is in thermodynamic equilibrium, ΦG is a minimum. This can be seen by considering that dΦG is zero if the volume is fixed and the temperature and chemical potential have stopped evolving. Landau free ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
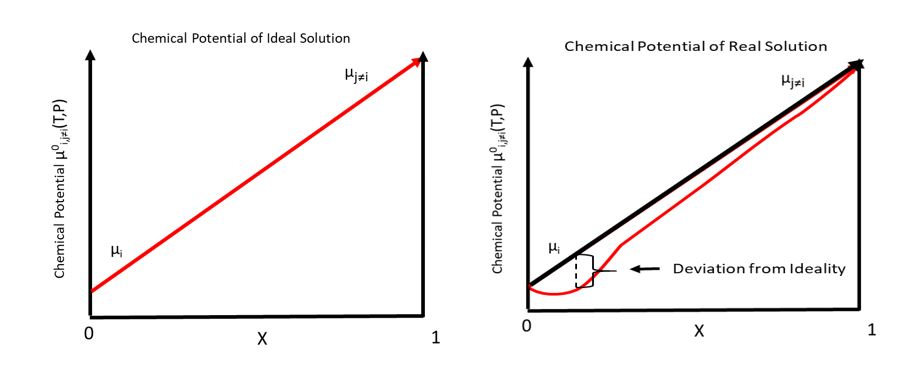
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Number
The particle number (or number of particles) of a thermodynamic system, conventionally indicated with the letter ''N'', is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle number is a dimensionless quantity. It is an extensive parameter, as it is directly proportional to the size of the system under consideration, and thus meaningful only for closed systems. A constituent particle is one that cannot be broken into smaller pieces at the scale of energy ''k·T'' involved in the process (where ''k'' is the Boltzmann constant and ''T'' is the temperature). For example, for a thermodynamic system consisting of a piston containing water vapour, the particle number is the number of water molecules in the system. The meaning of constituent particle, and thereby of particle number, is thus temperature-dependent. Determining the particle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exterior Derivative
On a differentiable manifold, the exterior derivative extends the concept of the differential of a function to differential forms of higher degree. The exterior derivative was first described in its current form by Élie Cartan in 1899. The resulting calculus, known as exterior calculus, allows for a natural, metric-independent generalization of Stokes' theorem, Gauss's theorem, and Green's theorem from vector calculus. If a differential -form is thought of as measuring the flux through an infinitesimal - parallelotope at each point of the manifold, then its exterior derivative can be thought of as measuring the net flux through the boundary of a -parallelotope at each point. Definition The exterior derivative of a differential form of degree (also differential -form, or just -form for brevity here) is a differential form of degree . If is a smooth function (a -form), then the exterior derivative of is the differential of . That is, is the unique -form such that for e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Equations
Gibbs or GIBBS is a surname and acronym. It may refer to: People * Gibbs (surname) Places * Gibbs (crater), on the Moon * Gibbs, Missouri, US * Gibbs, Tennessee, US * Gibbs Island (South Shetland Islands), Antarctica * 2937 Gibbs, an asteroid Science Mathematics and statistics * Gibbs phenomenon * Gibbs' inequality * Gibbs sampling Physics * Gibbs phase rule * Gibbs free energy * Gibbs entropy * Gibbs paradox * Gibbs–Helmholtz equation * Gibbs algorithm * Gibbs state * Gibbs-Marangoni effect * Gibbs phenomenon, an MRI artifact Organisations * Gibbs & Cox naval architecture firm * Gothenburg International Bioscience Business School * Gibbs College, several US locations * Gibbs Technologies, developer and manufacturer of amphibious vehicles * Gibbs High School (other), several schools of this name exist * Antony Gibbs & Sons, British trading company, established in London in 1802 Other uses * Gibbs SR, former name of the toothpaste Mentadent * Gibbs Stadium, Spar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetry Of Second Derivatives
In mathematics, the symmetry of second derivatives (also called the equality of mixed partials) refers to the possibility of interchanging the order of taking partial derivatives of a function :f\left(x_1,\, x_2,\, \ldots,\, x_n\right) of ''n'' variables without changing the result under certain conditions (see below). The symmetry is the assertion that the second-order partial derivatives satisfy the identity :\frac \left( \frac \right) \ = \ \frac \left( \frac \right) so that they form an ''n'' × ''n'' symmetric matrix, known as the function's Hessian matrix. This is sometimes known as Schwarz's theorem, Clairaut's theorem, or Young's theorem. In the context of partial differential equations it is called the Schwarz integrability condition. Formal expressions of symmetry In symbols, the symmetry may be expressed as: :\frac \left( \frac \right) \ = \ \frac \left( \frac \right) \qquad\text\qquad \frac \ =\ \frac . Another nota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Derivative
In mathematics, the total derivative of a function at a point is the best linear approximation near this point of the function with respect to its arguments. Unlike partial derivatives, the total derivative approximates the function with respect to all of its arguments, not just a single one. In many situations, this is the same as considering all partial derivatives simultaneously. The term "total derivative" is primarily used when is a function of several variables, because when is a function of a single variable, the total derivative is the same as the ordinary derivative of the function. The total derivative as a linear map Let U \subseteq \R^n be an open subset. Then a function f:U \to \R^m is said to be (totally) differentiable at a point a\in U if there exists a linear transformation df_a:\R^n \to \R^m such that :\lim_ \frac=0. The linear map df_a is called the (total) derivative or (total) differential of f at a. Other notations for the total derivative inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mnemonic
A mnemonic ( ) device, or memory device, is any learning technique that aids information retention or retrieval (remembering) in the human memory for better understanding. Mnemonics make use of elaborative encoding, retrieval cues, and imagery as specific tools to encode information in a way that allows for efficient storage and retrieval. Mnemonics aid original information in becoming associated with something more accessible or meaningful—which, in turn, provides better retention of the information. Commonly encountered mnemonics are often used for lists and in auditory form, such as short poems, acronyms, initialisms, or memorable phrases, but mnemonics can also be used for other types of information and in visual or kinesthetic forms. Their use is based on the observation that the human mind more easily remembers spatial, personal, surprising, physical, sexual, humorous, or otherwise "relatable" information, rather than more abstract or impersonal forms of informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Square
The thermodynamic square (also known as the thermodynamic wheel, Guggenheim scheme or Born square) is a mnemonic diagram attributed to Max Born and used to help determine thermodynamic relations. Born presented the thermodynamic square in a 1929 lecture. The symmetry of thermodynamics appears in a paper by F.O. Koenig. The corners represent common conjugate variables while the sides represent thermodynamic potentials. The placement and relation among the variables serves as a key to recall the relations they constitute. A mnemonic used by students to remember the Maxwell relations (in thermodynamics) is "Good Physicists Have Studied Under Very Fine Teachers", which helps them remember the order of the variables in the square, in clockwise direction. Another mnemonic used here is "Valid Facts and Theoretical Understanding Generate Solutions to Hard Problems", which gives the letter in the normal left-to-right writing direction. Both times A has to be identified with F, another com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is minim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |