|
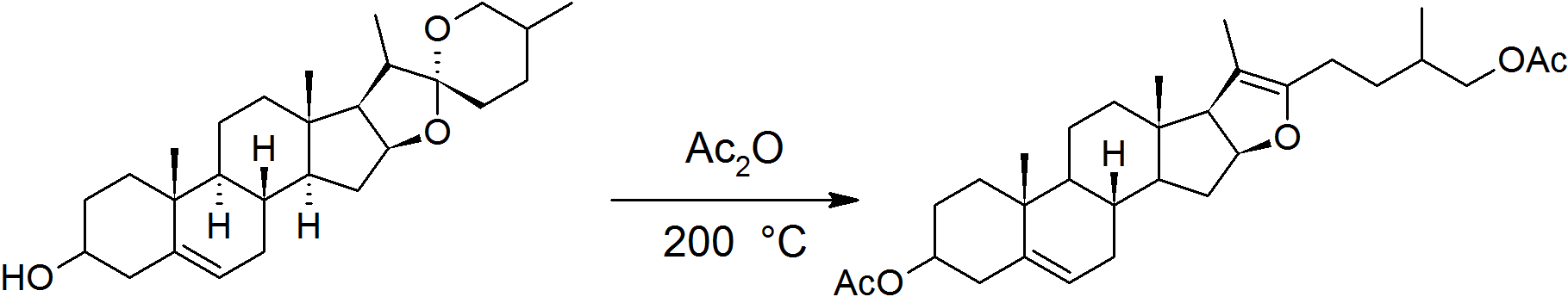
Marker Degradation
The Marker degradation is a three-step synthetic route in steroid chemistry developed by American chemist Russell Earl Marker in 1938–40. It is used for the production of cortisone and mammalian sex hormones (progesterone, estradiol, etc.) from plant steroids, and established Mexico as a world center for steroid production in the years immediately after World War II. The discovery of the Marker degradation allowed the production of substantial quantities of steroid hormones for the first time, and was fundamental in the development of the contraceptive pill and corticosteroid anti-inflammatory drugs. In 1999, the American Chemical Society and the Sociedad Química de México named the route as an International Historic Chemical Landmark. The first large-scale application of the route took place in 1943, when Russell Earl Marker collected 10 tons of yam tubers to synthesize of progesterone, which was the largest single amount of progesterone that had been produced by that t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fine Chemicals
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg (see the comparison of fine chemicals, commodities and specialties). The class of fine chemicals is subdivided either on the basis of the added value (building blocks, advanced intermediates or active ingredients), or the type of business transaction, namely standard or exclusive products. Fine chemicals are produced in limited volumes ( $10/kg) according to exacting specifications, mainly by traditional organic synthesis in multipurpose chemical plants. Biotechnical processes are gaining ground. Fine chemicals are used as starting materials for specialty chemicals, particularly pharmaceuticals, biopharmaceuticals and agrochemicals. Custom manufacturing fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioscorea
''Dioscorea'' is a genus of over 600 species of flowering plants in the family Dioscoreaceae, native throughout the tropical and warm temperate regions of the world. The vast majority of the species are tropical, with only a few species extending into temperate climates. It was named by the monk Charles Plumier after the ancient Greek physician and botanist Dioscorides. Description Wild Yam (''Dioscorea'') is a vine that is invasive, deciduous, and herbaceous. This species is native to Asia, though, in the U.S., it is commonly found in Florida. They can grow over in length. Wild yams are an important crop, as they have been used to prevent menstrual cramps, stomach cramps, and general pain for centuries. During the 1950s scientists found that the roots of wild yams contained diosgenin which is a plant-based estrogen; diosgenin is hypothesized to aid in chemical defense against herbivores. This was used to create the first birth control pills during the 60s. In addition, some '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north toward the East China Sea, Philippine Sea, and Taiwan in the south. Japan is a part of the Ring of Fire, and spans Japanese archipelago, an archipelago of List of islands of Japan, 6852 islands covering ; the five main islands are Hokkaido, Honshu (the "mainland"), Shikoku, Kyushu, and Okinawa Island, Okinawa. Tokyo is the Capital of Japan, nation's capital and largest city, followed by Yokohama, Osaka, Nagoya, Sapporo, Fukuoka, Kobe, and Kyoto. Japan is the List of countries and dependencies by population, eleventh most populous country in the world, as well as one of the List of countries and dependencies by population density, most densely populated and Urbanization by country, urbanized. About three-fourths of Geography of Japan, the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diosgenin
Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of ''Dioscorea'' wild yam species, such as the Kokoro. The sugar-free (aglycone) product of such hydrolysis, diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products. Sources It is present in detectable amounts in '' Costus speciosus'', '' Smilax menispermoidea'', ''Helicteres isora'', species of ''Paris'', ''Aletris'', ''Trigonella'', and ''Trillium'', and in extractable amounts from many species of ''Dioscorea'' – '' D. althaeoides'', '' D. colletti'', '' D. composita'', '' D. floribunda'', '' D. futschauensis'', '' D. gracillima'', '' D. hispida'', '' D. hypoglauca'', '' D. mexicana'', '' D. nipponica'', '' D. panthaica'', '' D. parviflora'', '' D. septemloba'', and '' D. zingiberensis''. Industrial uses Diosgenin is a precursor for several hormones, starting with the Marker ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trillium Erectum
''Trillium erectum'', the red trillium, also known as wake robin, purple trillium, bethroot, or stinking benjamin, is a species of flowering plant in the family (biology), family Melanthiaceae. The plant takes its common name "wake robin" by analogy with the European robin, which has a red breast heralding spring. Likewise ''Trillium erectum'' is a ephemeral plant, spring ephemeral whose life-cycle is synchronized with that of the forests in which it lives. It is native plant, native to the eastern United States and eastern Canada from northern Georgia (U.S. state), Georgia to Quebec and New Brunswick. Description ''Trillium erectum'' is a Perennial plant, perennial herbaceous plant that grows to about in height with a spread of . It can tolerate extreme cold in winter, surviving temperatures down to . Like all trilliums, its parts are in groups of three, with a 3-petalled flower above a whorl of pointed triple leaves. The petals are dark reddish brown, maroon, purple, pale ye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Decomposition
Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity (normal molecule, reaction intermediate, etc.) into two or more fragments. Chemical decomposition is usually regarded and defined as the exact opposite of chemical synthesis. In short, the chemical reaction in which two or more products are formed from a single reactant is called a decomposition reaction. The details of a decomposition process are not always well defined but some of the process is understood; much energy is needed to break bonds. Since all decomposition reactions break apart the bonds holding it together in order to produce into its simpler basic parts, the reactions would require some form of this energy in varying degrees. Because of this fundamental rule, it is known that most of these reactions are endothermic although exceptions do exist. The stability of a chemical compound is eventually limited when exposed to extreme environmental conditions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (havi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarsasapogenin
Sarsasapogenin is a steroidal sapogenin, that is the aglycosidic portion of a plant saponin. It is named after sarsaparilla (''Smilax'' sp.),. a family of climbing plants found in subtropical regions. It was one of the first sapogenins to be identified, and the first spirostan steroid to be identified as such.. The identification of the spirostan structure, with its ketone spiro acetal functionality, was fundamental in the development of the Marker degradation, which allowed the industrial production of progesterone and other sex hormones from plant steroids. Sarsasapogenin is unusual in that it has a ''cis''-linkage between rings A and B of the steroid nucleus, as opposed to the more usual ''trans''-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – sarsasapogenin 3β-glucosyltransferase – is found in several plants for the glycosylation of sarsasapogenin. The (''S'')-configuration at C-25 is also in cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |