|
Magnesiochromite
Chromite is a crystalline mineral composed primarily of iron(II) oxide and chromium(III) oxide compounds. It can be represented by the chemical formula of FeCr2O4. It is an oxide mineral belonging to the spinel group. The element magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4). A substitution of the element aluminium can also occur, leading to hercynite (FeAl2O4). Chromite today is mined particularly to make stainless steel through the production of ferrochrome (FeCr), which is an iron-chromium alloy. Chromite grains are commonly found in large mafic igneous intrusions such as the Bushveld in South Africa and India. Chromite is iron-black in color with a metallic luster, a dark brown streak and a hardness on the Mohs scale of 5.5. Properties Chromite minerals are mainly found in mafic-ultramafic igneous intrusions and are also sometimes found in metamorphic rocks. The chromite minerals occur in layered format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Minerals
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately. Simple oxides: *XO **Periclase group ***Periclase ***Manganosite **Zincite group ***Zincite *** Bromellite ***Tenorite ***Litharge * **Cuprite **Ice * **Hematite group ***Corundum ***Hematite ***Ilmenite * **Rutile group ***Rutile ***Pyrolusite *** Cassiterite ** Baddeleyite **Uraninite **Thorianite * **Spinel group ***Spinel ***Gahnite ***Magnetite ***Franklinite *** Chromite **Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite **Manganite ** Romanèchite **Goethite group: ***Diaspore ***Goethite Nickel–Strunz Classification -04- Oxides IMA-CNMNC proposes a new hierarchical scheme (Mills et al., 2009). This list uses it to mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lustre (mineralogy)
Lustre (British English) or luster (American English; see spelling differences) is the way light interacts with the surface of a crystal, rock, or mineral. The word traces its origins back to the Latin ''lux'', meaning "light", and generally implies radiance, gloss, or brilliance. A range of terms are used to describe lustre, such as ''earthy'', ''metallic'', ''greasy'', and ''silky''. Similarly, the term ''vitreous'' (derived from the Latin for glass, ''vitrum'') refers to a glassy lustre. A list of these terms is given below. Lustre varies over a wide continuum, and so there are no rigid boundaries between the different types of lustre. (For this reason, different sources can often describe the same mineral differently. This ambiguity is further complicated by lustre's ability to vary widely within a particular mineral species). The terms are frequently combined to describe intermediate types of lustre (for example, a "vitreous greasy" lustre). Some minerals exhibit unus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isostructural
Isostructural chemical compounds have similar chemical structures. "Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous substances crystallise in the same space group and have the same unit cell dimensions. The IUCR definitionIUCR Online Dictionary of CRYSTALLOGRAPHY, "Isostructural crystals"/ref> used by crystallographers is: Examples include: *I- Gold(I) bromide is isostructural with gold(I) chloride *Borazine is isostructural with benzene * Indium(I) bromide is isostructural with β-thallium(I) iodide and has a distorted rock salt structure. Many minerals are isostructural when they differ only in the nature of a cation. Compounds which are isoelectronic usually have similar chemical structures. For example, methane, CH4, and the ammonium ion, NH4+, are isoelectric and are isostructural as both have a tetrahedral structure. The C-H and N-H bond length ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism. Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks. The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ''ferrous-ferric oxide''. Properties In addition to igneous rocks, magnetite also occurs in sedimentary rocks, including banded iron formations and in lake and marine sediments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xieite
Xieite is an iron chromium oxide mineral with formula Fe2+Cr2O4. It is a member of the spinel group and a high pressure polymorph of chromite. It was discovered in samples taken from the Suizhou meteorite which fell in 1986 in the Zengdu District Zengdu () is a district of the city of Suizhou, Hubei province, People's Republic of China. Administrative divisions Four subdistricts: * Xicheng Subdistrict (), Dongcheng Subdistrict (), Nanjiao Subdistrict (), Beijiao Subdistrict () Five tow ... of China. References Oxide minerals Meteorite minerals Orthorhombic minerals Minerals in space group 63 Spinel group {{oxide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Euhedral And Anhedral
Euhedral crystals (also known as idiomorphic or automorphic crystals) are those that are well-formed, with sharp, easily recognised faces. The opposite is anhedral (also known as ''xenomorphic'' or ''allotriomorphic''): a rock with an anhedral texture is composed of mineral grains that have no well-formed crystal faces or cross-section shape in thin section. Anhedral crystal growth occurs in a competitive environment with no free space for the formation of crystal faces. An intermediate texture with some crystal face-formation is termed subhedral. Crystals that grow from cooling liquid magma typically do not form smooth faces or sharp crystal outlines. As magma cools, the crystals grow and eventually touch each other, preventing crystal faces from forming properly or at all. When snowflakes crystallize, they do not touch each other. Thus, snowflakes form euhedral, six-sided twinned crystals. In rocks, the presence of euhedral crystals may signify that they formed early in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troilite
Troilite is a rare iron sulfide mineral with the simple formula of FeS. It is the iron-rich endmember of the pyrrhotite group. Pyrrhotite has the formula Fe(1-x)S (x = 0 to 0.2) which is iron deficient. As troilite lacks the iron deficiency which gives pyrrhotite its characteristic magnetism, troilite is non-magnetic. Troilite can be found as a native mineral on Earth but is more abundant in meteorites, in particular, those originating from the Moon and Mars. It is among the minerals found in samples of the meteorite that struck Russia in Chelyabinsk on February 15th, 2013. Uniform presence of troilite on the Moon and possibly on Mars has been confirmed by the Apollo, Viking and Phobos space probes. The relative intensities of isotopes of sulfur are rather constant in meteorites as compared to the Earth minerals, and therefore troilite from Canyon Diablo meteorite is chosen as the international sulfur isotope ratio standard, the Canyon Diablo Troilite (CDT). Structure Troilit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
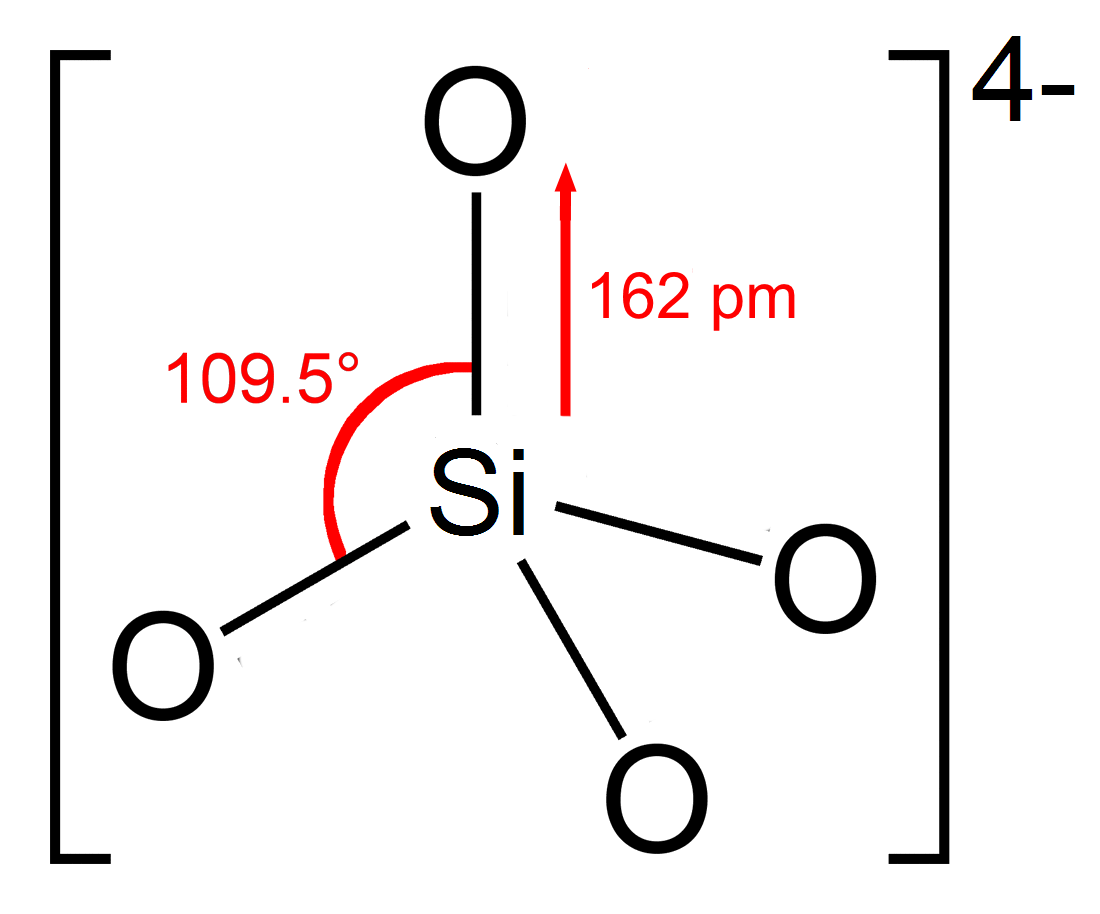
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose corner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Meteorite
Iron meteorites, also known as siderites, or ferrous meteorites, are a type of meteorite that consist overwhelmingly of an iron–nickel alloy known as meteoric iron that usually consists of two mineral phases: kamacite and taenite. Most iron meteorites originate from cores of planetesimals, with the exception of the IIE iron meteorite group The iron found in iron meteorites was one of the earliest sources of usable iron available to humans, due to the malleability and ductility of the meteoric iron, before the development of smelting that signaled the beginning of the Iron Age. Occurrence Although they are fairly rare compared to the stony meteorites, comprising only about 5.7% of witnessed falls, iron meteorites have historically been heavily over-represented in meteorite collections. This is due to several factors: * They are easily recognized as unusual even by laymen, as opposed to stony meteorites. Modern-day searches for meteorites in deserts and Antarctica yield a muc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metamorphic Rock
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock (protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, causing profound physical or chemical changes. During this process, the rock remains mostly in the solid state, but gradually recrystallizes to a new texture or mineral composition. The protolith may be an igneous, sedimentary, or existing metamorphic rock. Metamorphic rocks make up a large part of the Earth's crust and form 12% of the Earth's land surface. They are classified by their protolith, their chemical and mineral makeup, and their texture. They may be formed simply by being deeply buried beneath the Earth's surface, where they are subject to high temperatures and the great pressure of the rock layers above. They can also form from tectonic processes such as continental collisions, which cause horizontal pressure, friction, and distorti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |