|
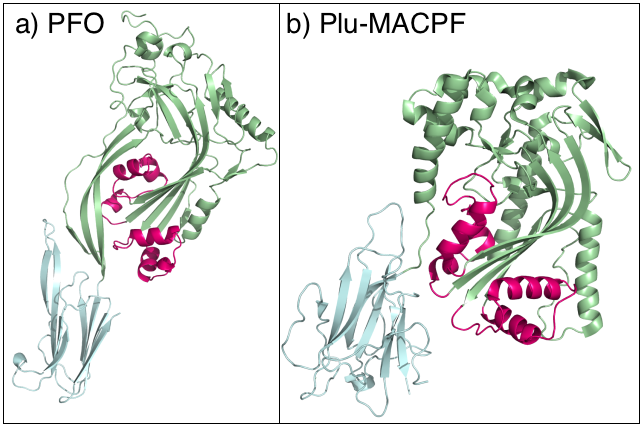
MACPF
The Membrane Attack Complex/Perforin (MACPF) superfamily, sometimes referred to as the MACPF/CDC superfamily, is named after a domain that is common to the membrane attack complex (MAC) proteins of the complement system (C6, C7, C8α, C8β and C9) and perforin (PF). Members of this protein family are pore-forming toxins (PFTs). In eukaryotes, MACPF proteins play a role in immunity and development. Archetypal members of the family are complement C9 and perforin, both of which function in human immunity. C9 functions by punching holes in the membranes of Gram-negative bacteria. Perforin is released by cytotoxic T cells and lyses virally infected and transformed cells. In addition, perforin permits delivery of cytotoxic proteases called granzymes that cause cell death. Deficiency of either protein can result in human disease. Structural studies reveal that MACPF domains are related to cholesterol-dependent cytolysins (CDCs), a family of pore forming toxins previously thought ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleurotolysin
PleurotolysinTC# 1.C.97.1.1, a sphingomyelin-specific cytolysin. Its A (17 kDaQ8X1M9 and B (59 kDaQ5W9E8 components are assembled into a transmembrane pore complex. The Pleurotolysin Pore-Forming (Pleurotolysin) FamilyTC# 1.C.97 is a family of pore forming proteins belonging to the MACPF superfamily. Function Proteins with membrane-attack complex/perforin (MACPF) domains have a variety of biological roles, including defense and attack, organismal development, and cell adhesion and signaling. The distribution of these proteins in fungi appears to be restricted to some Pezizomycotina and Basidiomycota species only, in correlation with the aegerolysinsPF06355. These two protein groups coincide in only a few species, and they operate as cytolytic bi-component pore-forming agents. Representative proteins include pleurotolysin B, which has a MACPF domain, the aegerolysin-like protein pleurotolysin A, and the very similar ostreolysin ATC# 1.C.97.3.2 that has been purified from oyster mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pore Forming Toxins
Pore-forming proteins (PFTs, also known as pore-forming toxins) are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic (i.e., they kill cells), as they create unregulated pores in the membrane of targeted cells. Types PFTs can be divided into two categories, depending on the alpha-helical or beta-barrel architecture of their transmembrane channel that can consist either of * Alpha-pore-forming toxins ** e.g., Haemolysin E family, actinoporins, Corynebacterial porin B, Cytolysin A of ''E. coli''. * Beta-barrel pore-forming toxins ** e.g. α-hemolysin (Fig 1), PVL – Panton-Valentine leukocidin, various insecticidal toxins. Other categories: * Large beta-barrel pore-forming toxins ** MACPF and Cholesterol-dependent cytolysins (CDCs), gasdermin * Binary toxins ** e.g., Anthrax toxin, Pleurotolysi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement Membrane Attack Complex
The membrane attack complex (MAC) or terminal complement complex (TCC) is a complex of proteins typically formed on the surface of pathogen cell membranes as a result of the activation of the host's complement system, and as such is an effector of the immune system. Antibody-mediated complement activation leads to MAC deposition on the surface of infected cells. Assembly of the MAC leads to pores that disrupt the cell membrane of target cells, leading to cell lysis and death. The MAC is composed of the complement components C5b, C6, C7, C8 and several C9 molecules. A number of proteins participate in the assembly of the MAC. Freshly activated C5b binds to C6 to form a C5b-6 complex, then to C7 forming the C5b-6-7 complex. The C5b-6-7 complex binds to C8, which is composed of three chains (alpha, beta, and gamma), thus forming the C5b-6-7-8 complex. C5b-6-7-8 subsequently binds to C9 and acts as a catalyst in the polymerization of C9. Structure and function MAC is composed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement Component 9
Complement component 9 (C9) is a MACPF protein involved in the complement system, which is part of the innate immune system. Once activated, about 12-18 molecules of C9 polymerize to form pores in target cell membranes, causing lysis and cell death. C9 is one member of the complement membrane attack complex (MAC), which also includes complement components C5b, C6, C7 and C8. The formation of the MAC occurs through three distinct pathways: the classical, alternative, and lectin pathways. Pore formation by C9 is an important way that bacterial cells are killed during an infection, and the target cell is often covered in multiple MACs. The clinical impact of a deficiency in C9 is an infection with the gram-negative bacterium ''Neisseria meningitidis.'' Structure C9 genes include 11 exons and 10 introns An intron is any nucleotide sequence within a gene that is not expressed or operative in the final RNA product. The word ''intron'' is derived from the term ''intragenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol-dependent Cytolysin
The thiol-activated Cholesterol-dependent Cytolysin (CDC) familyTC# 1.C.12 is a member of the MACPF superfamily. Cholesterol dependent cytolysins are a family of β-barrel pore-forming exotoxins that are secreted by gram-positive bacteria. CDCs are secreted as water-soluble monomers of 50-70 kDa, that when bound to the target cell, form a circular homo-oligomeric complex containing as many as 40 (or more) monomers. Through multiple conformational changes, the β-barrel transmembrane structure (~250 Å in diameter depending on the toxin) is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though the presence of cholesterol is not required by all CDCs for binding. For example, intermedilysin (ILYTC# 1.C.12.1.5 secreted by '' Streptococcus intermedius'' will bind only to target membranes containing a specific protein receptor, independent of the presence of cholesterol, but cholesterol is required b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol-dependent Cytolysin
The thiol-activated Cholesterol-dependent Cytolysin (CDC) familyTC# 1.C.12 is a member of the MACPF superfamily. Cholesterol dependent cytolysins are a family of β-barrel pore-forming exotoxins that are secreted by gram-positive bacteria. CDCs are secreted as water-soluble monomers of 50-70 kDa, that when bound to the target cell, form a circular homo-oligomeric complex containing as many as 40 (or more) monomers. Through multiple conformational changes, the β-barrel transmembrane structure (~250 Å in diameter depending on the toxin) is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though the presence of cholesterol is not required by all CDCs for binding. For example, intermedilysin (ILYTC# 1.C.12.1.5 secreted by '' Streptococcus intermedius'' will bind only to target membranes containing a specific protein receptor, independent of the presence of cholesterol, but cholesterol is required b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perforin
Perforin-1 is a protein that in humans is encoded by the ''PRF1'' gene and the ''Prf1'' gene in mice. Function Perforin is a pore forming cytolytic protein found in the granules of cytotoxic T lymphocytes (CTLs) and natural killer cells (NK cells). Upon degranulation, perforin molecules translocate to the target cell with the help of calreticulin, which works as a chaperone protein to prevent perforin from degrading. Perforin then binds to the target cell's plasma membrane via membrane phospholipids while phosphatidylcholine binds calcium ions to increase perforin's affinity to the membrane. Perforin oligomerises in a Ca2+ dependent manner to form pores on the target cell. The pore formed allows for the passive diffusion of a family of pro-apoptotic proteases, known as the granzymes, into the target cell. The lytic membrane-inserting part of perforin is the MACPF domain. This region shares homology with cholesterol-dependent cytolysins from Gram-positive bacteria. Perforin has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perforin
Perforin-1 is a protein that in humans is encoded by the ''PRF1'' gene and the ''Prf1'' gene in mice. Function Perforin is a pore forming cytolytic protein found in the granules of cytotoxic T lymphocytes (CTLs) and natural killer cells (NK cells). Upon degranulation, perforin molecules translocate to the target cell with the help of calreticulin, which works as a chaperone protein to prevent perforin from degrading. Perforin then binds to the target cell's plasma membrane via membrane phospholipids while phosphatidylcholine binds calcium ions to increase perforin's affinity to the membrane. Perforin oligomerises in a Ca2+ dependent manner to form pores on the target cell. The pore formed allows for the passive diffusion of a family of pro-apoptotic proteases, known as the granzymes, into the target cell. The lytic membrane-inserting part of perforin is the MACPF domain. This region shares homology with cholesterol-dependent cytolysins from Gram-positive bacteria. Perforin has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photorhabdus Luminescens
''Photorhabdus luminescens'' (previously called ''Xenorhabdus luminescens'') is a Gammaproteobacterium of the family Morganellaceae, and is a lethal pathogen of insects. It lives in the gut of an entomopathogenic nematode of the family Heterorhabditidae. When the nematode infects an insect, ''P. luminescens'' is released into the blood stream and rapidly kills the insect host (within 48 hours) by producing toxins, such as the high molecular weight insecticidal protein complex Tca. ''P. luminescens'' also produces a proteic toxin through the expression of a single gene called ''makes caterpillars floppy'' (mcf). It also secretes enzymes which break down the body of the infected insect and bioconvert it into nutrients which can be used by both nematode and bacteria. In this way, both organisms gain enough nutrients to replicate (or reproduce in the case of the nematode) several times. The bacteria enter the nematode progeny as they develop. 3,5-Dihydroxy-4-isopropyl-trans-stilb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heliocidaris Erythrogramma
''Heliocidaris'' is a genus of sea urchins, part of the familia Echinometridae. Characteristics This genus is typical of west Pacific Ocean (Japan to New Zealand), in particular in Australia. Some species are edible. List of species This genus contains 6 extant species and 1 fossil : *''Heliocidaris australiae'' (Alexander Agassiz, A. Agassiz, 1872) *''Heliocidaris bajulus'' (Dartnall, 1972) *''Heliocidaris crassispina'' (Alexander Agassiz, A. Agassiz, 1863) *''Heliocidaris erythrogramma'' (Achille Valenciennes, Valenciennes, 1846) *''Heliocidaris ludbrookae'' Philip, 1965 † *''Heliocidaris robertsi'' Lindley, 2004 *''Heliocidaris tuberculata'' (Jean-Baptiste Lamarck, Lamarck, 1816) Image:Heliocidaris erythrogramma P1142284.JPG, ''Heliocidaris erythrogramma'' Image:Heliocidaris tuberculata.jpg, ''Heliocidaris tuberculata'' References Echinometridae {{echinoidea-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malarial
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin ten to fifteen days after being bitten by an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria. Malaria is caused by single-celled microorganisms of the ''Plasmodium'' group. It is spread exclusively through bites of infected ''Anopheles'' mosquitoes. The mosquito bite introduces the parasites from the mosquito's saliva into a person's blood. The parasites travel to the liver where they mature and reproduce. Five species of ''Plasmodium'' can infect and be sprea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astrotactin
Astrotactin (Astn1) is a glycoprotein expressed on migrating neurons that favors adhesion to glia Glia, also called glial cells (gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. They maintain homeostasis, form myel ... and migration. It is involved in regulation of adhesion during the radial migration of neurons in the developing CNS. Its expression is limited to cells in the cortex and cerebellum. * Alberts, B.(1994) Molecular Biology of the Cell * Price et al., (2011) Building Brains {{protein-stub Glycoproteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |