|
Mucobromic Acid
Mucobromic acid is an organic compound that consists of a dibrominated alkene with aldehyde and carboxylic acid functional groups. It easily tautomerizes to a furanone hemiacetal form. This compound, and the analogous mucochloric acid (CAS #87-56-9), form the group of known mucohalic acids. The bromide appears to behave similarly to the more heavily studied chloride. Synthesis and structure Mucobromic acid can be synthesized by bromination of furfural via an oxidation/decarboxylation process: :C4H4OCHO + 3 Br2 + 3 H2O → C2Br2CHO(CO2H) + CO2 + 8 HBr Mucobromic acid exists as a mixture acyclic and cyclic isomers. The compound can be reduced using sodium borohydride to give the lactone. Hydrolysis under basic conditions of either the chloro or bromo compound involves substitution of the halide adjacent to the acid. The resulting mucoxyhalic acids exist as a mixture of keto and enol forms. The reaction occurs via a conjugate addition/ elimination of the alkene–aldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula Na BH4. This white solid, usually encountered as an aqueous basic solution, is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis. The compound was discovered in the 1940s by H. I. Schlesinger, who led a team seeking volatile uranium compounds.Hermann I Schlesinger and Herbert C Brown (1945)Preparation of alkali metal compounds. US Patent 2461661. Granted on 1949-02-15; expired on 1966-02-15. Results of this wartime research were declassified and published in 1953. Properties The compound is soluble in alcohols, certain ethers, and water, although it slowly hydrolyzes. Sodium borohydride is an odorless white to gray-white microcrystalline powder that often forms lumps. It can be purified by recrystallization from warm (50 °C) diglyme. Sodium borohydride is soluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine
Adenosine ( symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert the rhyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
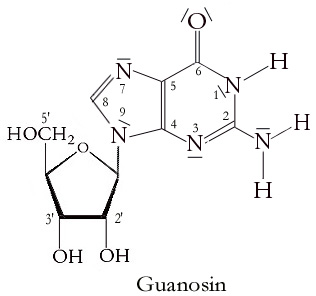
Guanosine
Guanosine (symbol G or Guo) is a purine nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These forms play important roles in various biochemical processes such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction (cGMP). When guanine is attached by its N9 nitrogen to the C1 carbon of a deoxyribose ring it is known as deoxyguanosine. Physical and chemical properties Guanosine is a white, crystalline powder with no odor and mild saline taste. It is very soluble in acetic acid, slightly soluble in water, insoluble in ethanol, diethyl ether, benzene and chloroform. Functions Guanosine is required for an RNA splicing reaction in mRNA, when a "self-splicing" intron removes itself from the mRNA messag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genotoxins
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis. To assay for genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C Pi bond''). The specifics of the reaction are as follows: * E2 is a single step elimination, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles. Reaction mechanism Conjugate addition is the vinylogous counterpart of direct nucleophilic addition. A nucleophile reacts with a α,β-unsaturated carbonyl compound in the β position. The negative charge carried by the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keto–enol Tautomerism
In organic chemistry, alkenols (shortened to enols) are a type of Functional group, reactive structure or chemical intermediate, intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The terms ''enol'' and ''alkenol'' are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of Alcohol (chemistry), alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, . When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name ''furfural'' comes from the Latin word , meaning bran, referring to its usual source. Furfural is only derived from lignocellulosic biomass, i.e., its origin is non-food or non-coal/oil based. Aside from ethanol, acetic acid, and sugar, it is one of the oldest renewable chemicals. It is also found in many processed foods and beverages. History Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct of formic acid synthesis. In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |