|
Moseley's Law
Moseley's law is an empirical law concerning the characteristic X-rays emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913–1914. Until Moseley's work, "atomic number" was merely an element's place in the periodic table and was not known to be associated with any measurable physical quantity. In brief, the law states that the square root of the frequency of the emitted X-ray is approximately proportional to the atomic number: \sqrt \nu \varpropto Z . History The History of the periodic table, historic periodic table was roughly ordered by increasing Standard atomic weight, atomic ''weight'', but in a few famous cases the physical properties of two elements suggested that the heavier ought to precede the lighter. An example is cobalt having the atomic weight of 58.9 and nickel having the atomic weight of 58.7. Henry Moseley and other physicists used Bragg's law, X-ray diffraction to study the elements, and the results of their e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moseley Step Ladder
Moseley ( ') is an affluent suburb in south Birmingham, England, south of the city centre. It is located within the eponymous Moseley ward of the constituency of Birmingham Hall Green and Moseley (UK Parliament constituency), Hall Green and Moseley in the Ceremonial counties of England, ceremonial county of the West Midlands (county), West Midlands. It Historic counties of England, historically lay within Worcestershire, abutting the county border with Warwickshire. History Moseley was listed as a settlement within the manor of Bromsgrove in the Domesday Book of 1086 as ''Museleie'', from the Anglo-Saxon language, Anglo-Saxon ''mús'' (mouse) + ''leáh'' (lea, meadow), which translates as either 'mouse clearing' or 'mouse-sized (i.e. small) clearing'. St. Mary's Church, Moseley, St Mary's Church, Moseley was licensed by the Bishop of Worcester (authorised by Pope Innocent VII) in February 1405. St Anne's Church, Moseley was opened in 1874 for the now extinct parish of Park ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Line Fitting
Line fitting is the process of constructing a straight line that has the best fit to a series of data points. Several methods exist, considering: *Vertical distance: Simple linear regression *Resistance to outliers: Robust simple linear regression *Perpendicular distance: Orthogonal regression (this is not scale-invariant i.e. changing the measurement units leads to a different line.) *Weighted geometric distance: Deming regression *Scale invariant approach: Major axis regression This allows for measurement error in both variables, and gives an equivalent equation if the measurement units are altered. See also *Linear least squares *Linear segmented regression *Linear trend estimation *Polynomial regression *Regression dilution Regression dilution, also known as regression attenuation, is the biasing of the linear regression slope towards zero (the underestimation of its absolute value), caused by errors in the independent variable. Consider fitting a straight line ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
History Of Physics
Physics is a branch of science in which the primary objects of study are matter and energy. These topics were discussed across many cultures in ancient times by philosophers, but they had no means to distinguish causes of natural phenomena from superstitions. The Scientific Revolution of the 17th century, especially the discovery of the law of gravity, began a process of knowledge accumulation and specialization that gave rise to the field of physics. Mathematical advances of the 18th century gave rise to classical mechanics, and the increased used of the experimental method led to new understanding of thermodynamics. In the 19th century, the basic laws of electromagnetism and statistical mechanics were discovered. At the beginning of the 20th century, physics was transformed by the discoveries of quantum mechanics, relativity, and atomic theory. Physics today may be divided loosely into classical physics and modern physics. Ancient history Elements of what became phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eponymous Laws Of Physics
An eponym is a noun after which or for which someone or something is, or is believed to be, named. Adjectives derived from the word ''eponym'' include ''eponymous'' and ''eponymic''. Eponyms are commonly used for time periods, places, innovations, biological nomenclature, astronomical objects, works of art and media, and tribal names. Various orthographic conventions are used for eponyms. Usage of the word The term ''eponym'' functions in multiple related ways, all based on an explicit relationship between two named things. ''Eponym'' may refer to a person or, less commonly, a place or thing for which someone or something is, or is believed to be, named. ''Eponym'' may also refer to someone or something named after, or believed to be named after, a person or, less commonly, a place or thing. A person, place, or thing named after a particular person share an eponymous relationship. In this way, Elizabeth I of England is the eponym of the Elizabethan era, but the Elizabethan e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Neutron
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford developed a crude Rutherford model, model of the atom, based on the Geiger–Marsden experiment, gold foil experiment of Hans Geiger and Ernest Marsden. In this model, atoms had their mass and Electric charge, positive electric charge concentrated in a very small atomic nucleus, nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be (approximately) Multiple (mathematics), integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus.Byrne, J. ''Neutrons, Nuclei, and Matter'', Dover Publications, Mineola, New York, 2011, Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auger Electron Spectroscopy
A Hanford scientist uses an Auger electron spectrometer to determine the elemental composition of surfaces. Auger electron spectroscopy (AES; pronounced in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. It is a form of electron spectroscopy that relies on the Auger effect, based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal ''Zeitschrift für Physik'' in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in X-ray spectroscopy data. Since 195 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Law
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain elements when grouped by period and/or group. They were discovered by the Russian chemist Dmitri Mendeleev in 1863. Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, nucleophilicity, electrophilicity, valency, nuclear charge, and metallic character. Mendeleev built the foundation of the periodic table. Mendeleev organized the elements based on atomic weight, leaving empty spaces where he believed undiscovered elements would take their places. Mendeleev's discovery of this trend allowed him to predict the existence and properties of three unknown elements, which were later discovered by other chemists and named gallium, scandium, and germanium. English physicist Henry Moseley discovered that organizing the elements by atomic number instead of atomic weight would naturally group elements with simi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Special Relativity
In physics, the special theory of relativity, or special relativity for short, is a scientific theory of the relationship between Spacetime, space and time. In Albert Einstein's 1905 paper, Annus Mirabilis papers#Special relativity, "On the Electrodynamics of Moving Bodies", the theory is presented as being based on just Postulates of special relativity, two postulates: # The laws of physics are Invariant (physics), invariant (identical) in all Inertial frame of reference, inertial frames of reference (that is, Frame of reference, frames of reference with no acceleration). This is known as the principle of relativity. # The speed of light in vacuum is the same for all observers, regardless of the motion of light source or observer. This is known as the principle of light constancy, or the principle of light speed invariance. The first postulate was first formulated by Galileo Galilei (see ''Galilean invariance''). Background Special relativity builds upon important physics ide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
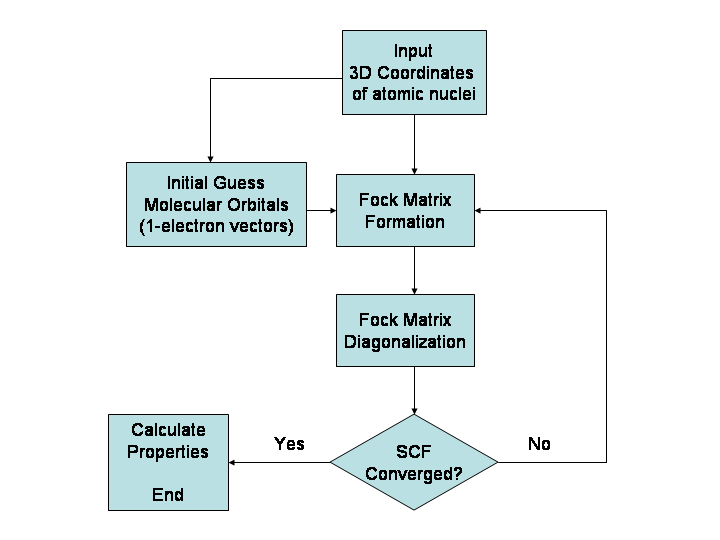
Hartree–Fock Method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The method is named after Douglas Hartree and Vladimir Fock. The Hartree–Fock method often assumes that the exact ''N''-body wave function of the system can be approximated by a single Slater determinant (in the case where the particles are fermions) or by a single permanent (in the case of bosons) of ''N'' spin-orbitals. By invoking the variational method, one can derive a set of ''N''-coupled equations for the ''N'' spin orbitals. A solution of these equations yields the Hartree–Fock wave function and energy of the system. Hartree–Fock approximation is an instance of mean-field theory, where neglecting higher-order fluctuations in order parameter allows interaction terms to be replaced with quadratic terms, obtaining exactly solvable Hamiltonians. Especially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planck Constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a matter wave equals the Planck constant divided by the associated particle momentum. The constant was postulated by Max Planck in 1900 as a proportionality constant needed to explain experimental black-body radiation. Planck later referred to the constant as the "quantum of Action (physics), action". In 1905, Albert Einstein associated the "quantum" or minimal element of the energy to the electromagnetic wave itself. Max Planck received the 1918 Nobel Prize in Physics "in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta". In metrology, the Planck constant is used, together with other constants, to define the kilogram, the SI unit of mass. The SI units are defined in such a way that, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Of An Electron
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . In SI units, the coulomb is defined such that the value of the elementary charge is exactly or 160.2176634 zeptocoulombs (zC). Since the 2019 revision of the SI, the seven SI base units are defined in terms of seven fundamental physical constants, of which the elementary charge is one. In the centimetre–gram–second system of units (CGS), the corresponding quantity is . Robert A. Millikan and Harvey Fletcher's oil drop experiment first directly measured the magnitude of the elementary charge in 1909, differing from the modern accepted value by just 0.6%. Under assumptions of the then-disputed atomic theory, the elementary charge had also been indirectly inferred to ~3% accuracy from blackbody spectr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Of An Electron
In particle physics, the electron mass (symbol: ) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about or about , which has an energy-equivalent of about or about . Terminology The term "rest mass" is sometimes used because in special relativity the mass of an object can be said to increase in a frame of reference that is moving relative to that object (or if the object is moving in a given frame of reference). Most practical measurements are carried out on moving electrons. If the electron is moving at a relativistic velocity, any measurement must use the correct expression for mass. Such correction becomes substantial for electrons accelerated by voltages of over . For example, the relativistic expression for the total energy, , of an electron moving at speed is E = \gamma m_\mathrm c^2 , where * is the speed of light; * is the Lorentz factor, \gamma = 1/\sqr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |