|
Molybdocene Dihydride
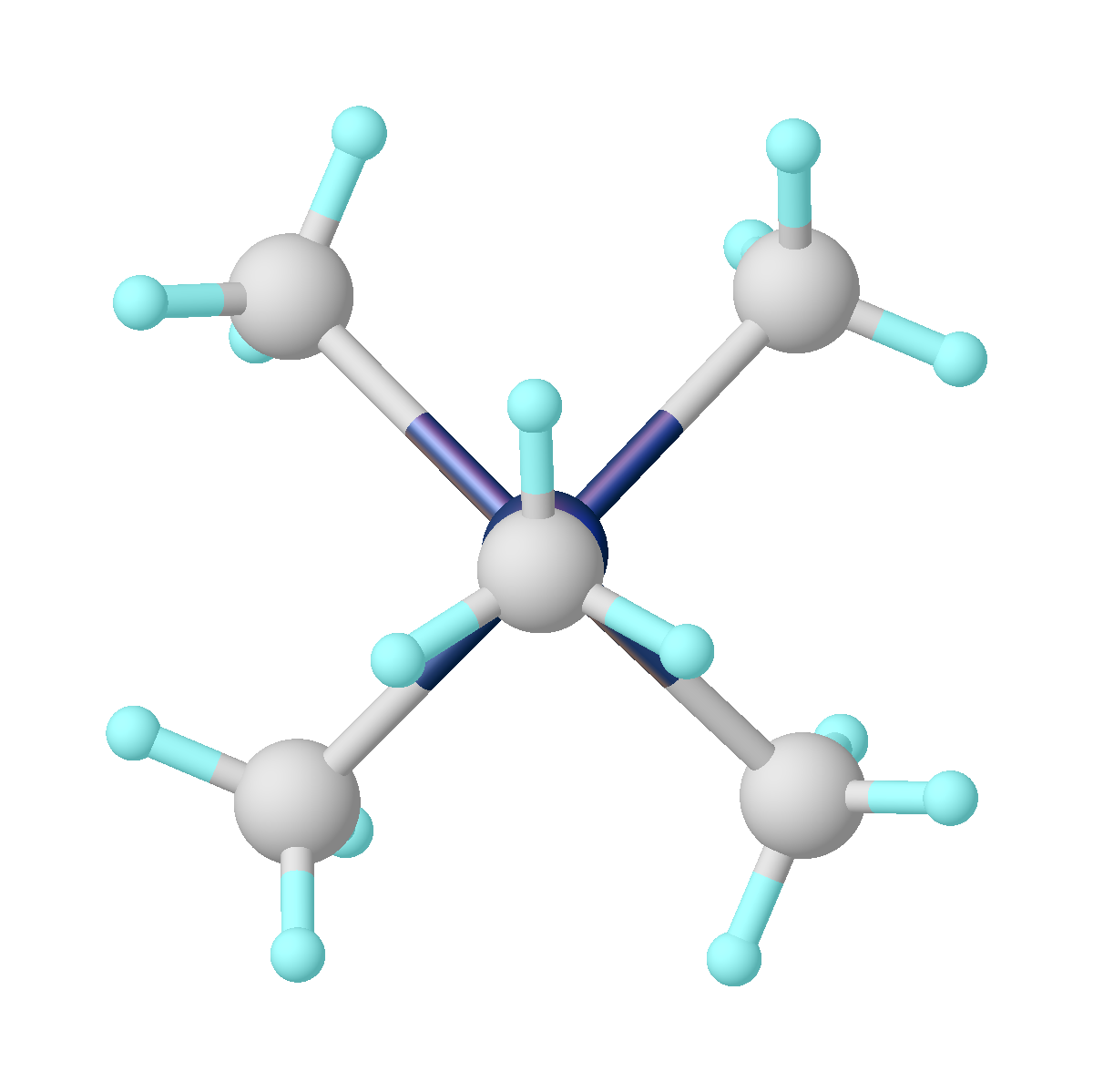
Molybdocene dihydride is the organomolybdenum compound with the formula ( η5-C5H5)2MoH2. Commonly abbreviated as Cp2MoH2, it is a yellow air-sensitive solid that dissolves in some organic solvents. The compound is prepared by combining molybdenum pentachloride, sodium cyclopentadienide, and sodium borohydride. The dihydride converts to molybdocene dichloride upon treatment with chloroform Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various .... The compound adopts a "clamshell" structure where the Cp rings are not parallel.K. Prout, T. S. Cameron, R. A. Forder, and in parts S. R. Critchley, B. Denton and G. V. Rees "The crystal and molecular structures of bent bis-π-cyclopentadienyl-metal complexes: (a) bis-π-cyclopentadienyldibromorhenium(V) tetrafluoroborate, (b) bis-π-cyclopen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomolybdenum Compound
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common. Mo(0) and more reduced states Molybdenum hexacarbonyl is the precursor to many substituted derivatives. It reacts with organolithium reagents to give anionic acyls which can be O-alkylated to give Fischer carbenes. 144px, Structure of (mesitylene)molybdenum tricarbonyl. Mo(CO)6 reacts with arenes to give piano-stool complexes such as (mesitylene)molybdenum tricarbonyl. Cycloheptatrienemolybdenum tricarbonyl, which is related to (arene)Mo(CO)3, reacts with trityl salts to give the cycloheptatrienyl complex: :(C7H8)Mo(CO)3 + (C6H5)3C+ → C7H7)Mo(CO)3sup>+ + (C6H5)3CH file:CHTMo(CO)3.png, 144px, Structure of Cycloheptatrienemolybdenum tricarbonyl. Reduction of Mo(CO)6 gives [Mo(CO)5]2− which is formally M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Orgel, and Rich described the structure of the "sandwich complex" ferrocene by X-ray crystallography where an iron atom is ''"sandwiched"'' between two parallel cyclopent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum Pentachloride
Molybdenum(V) chloride is the inorganic compound with the empirical formula . This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents. Structure Usually called molybdenum pentachloride, it is in fact partly a dimer with the molecular formula . In the dimer, each molybdenum has local octahedral symmetry and two chlorides bridge between the molybdenum centers. A similar structure is also found for the pentachlorides of W, Nb and Ta. In the gas phase and partly in solution, the dimers partially dissociate to give a monomeric . The monomer is paramagnetic, with one unpaired electron per Mo center, reflecting the fact that the formal oxidation state is +5, leaving one valence electron on the metal center. Preparation and properties is prepared by chlorination of Mo metal but also chlorination of . The unstable hexachloride is not produced in this way. is reduced by acetonitrile to afford an ora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities. Preparation Sodium cyclopentadienide is commercially available as a solution in THF. It is prepared by treating cyclopentadiene with sodium: : The conversion can be conducted by heating a suspension of molten sodium in dicyclopentadiene.Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878. In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring. Sodium hydride is a convenient base: : In early work, Grignard reagents were used as bases. With a p''K''a of 15, cyclope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula Na BH4. This white solid, usually encountered as an aqueous basic solution, is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis. The compound was discovered in the 1940s by H. I. Schlesinger, who led a team seeking volatile uranium compounds.Hermann I Schlesinger and Herbert C Brown (1945)Preparation of alkali metal compounds. US Patent 2461661. Granted on 1949-02-15; expired on 1966-02-15. Results of this wartime research were declassified and published in 1953. Properties The compound is soluble in alcohols, certain ethers, and water, although it slowly hydrolyzes. Sodium borohydride is an odorless white to gray-white microcrystalline powder that often forms lumps. It can be purified by recrystallization from warm (50 °C) diglyme. Sodium borohydride is soluble in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdocene Dichloride
Molybdocene dichloride is the organomolybdenum compound with the formula ( η5-C5H5)2MoCl2 and IUPAC name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture-sensitive powder. In the research laboratory, it is used to prepare many derivatives. Preparation and structure The compound is prepared from molybdocene dihydride Molybdocene dihydride is the organomolybdenum compound with the formula ( η5-C5H5)2MoH2. Commonly abbreviated as Cp2MoH2, it is a yellow air-sensitive solid that dissolves in some organic solvents. The compound is prepared by combining molybde ... by treatment with chloroform: :(C5H5)2MoH2 + 2 CHCl3 → (C5H5)2MoCl2 + 2 CH2Cl2 The compound adopts a "clamshell" structure where the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 130.6°. The two chloride ligands are cis, the Cl-Mo-Cl angle of 82° being narrower than in niobocene dichloride (85.6°), which in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-cente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocenes
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to p2ZrCH3sup>+ catalyze olefin polymerization. Some metallocenes consist of metal plus two cyclooctatetraenide anions (, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others). Metallocenes are a subset of a broader class of compounds called sandwich compounds. In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically stabilized. Here they are shown in a staggered conformation. Hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomolybdenum Compounds
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common. Mo(0) and more reduced states Molybdenum hexacarbonyl is the precursor to many substituted derivatives. It reacts with organolithium reagents to give anionic acyls which can be O-alkylated to give Fischer carbenes. 144px, Structure of (mesitylene)molybdenum tricarbonyl. Mo(CO)6 reacts with arenes to give piano-stool complexes such as (mesitylene)molybdenum tricarbonyl. Cycloheptatrienemolybdenum tricarbonyl, which is related to (arene)Mo(CO)3, reacts with trityl salts to give the cycloheptatrienyl complex: :(C7H8)Mo(CO)3 + (C6H5)3C+ → C7H7)Mo(CO)3sup>+ + (C6H5)3CH file:CHTMo(CO)3.png, 144px, Structure of Cycloheptatrienemolybdenum tricarbonyl. Reduction of Mo(CO)6 gives [Mo(CO)5]2− which is formally M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Complexes
{{Chemistry index ...
Cyclopentadienyl can refer to *Cyclopentadienyl anion, or cyclopentadienide, ** Cyclopentadienyl ligand * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also *Pentadienyl In organic chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula , where ''z'' = 0, −1, +1, respectively. Organometallic chemistry In organometallic chemistry, the pentadienyl anion is a ligand, the acyclic ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |