|
Molten Carbonate Fuel Cell
Molten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above. Molten carbonate fuel cells (MCFCs) were developed for natural gas, biogas (produced as a result of anaerobic digestion or biomass gasification), and coal- based power plants for electrical utility, industrial, and military applications. MCFCs are high-temperature fuel cells that use an electrolyte composed of a molten carbonate salt mixture suspended in a porous, chemically inert ceramic matrix of beta-alumina solid electrolyte (BASE). Since they operate at extremely high temperatures of 650 °C (roughly 1,200 °F) and above, non-precious metals can be used as catalysts at the anode and cathode, reducing costs. Improved efficiency is another reason MCFCs offer significant cost reductions over phosphoric acid fuel cells (PAFCs). Molten carbonate fuel cells can reach efficiencies approaching 60%, considerably higher than the 37–42% efficiencie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California, Irvine
The University of California, Irvine (UCI or UC Irvine) is a Public university, public Land-grant university, land-grant research university in Irvine, California, United States. One of the ten campuses of the University of California system, UCI offers 87 undergraduate degrees and 129 graduate and professional degrees, and roughly 30,000 undergraduates and 7,000 graduate students were enrolled at UCI as of Fall 2024. The university is Carnegie Classification of Institutions of Higher Education, classified among "R1: Doctoral Universities – Very high research activity" and had $609.6 million in research and development expenditures in 2023, ranking it 56th nationally. UCI became a member of the Association of American Universities in 1996. The university administers the UC Irvine Medical Center, a large teaching hospital in Orange, California, Orange, and UC Irvine Health Sciences, its affiliated health sciences system; the University of California, Irvine, Arboretum; and a po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fossil Fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geological formations. Reservoirs of such compound mixtures, such as coal, petroleum and natural gas, can be extracted and burnt as fuel for human consumption to provide energy for direct use (such as for cooking, heating or lighting), to power heat engines (such as steam or internal combustion engines) that can propel vehicles, or to generate electricity via steam turbine generators. Some fossil fuels are further refined into derivatives such as kerosene, gasoline and diesel, or converted into petrochemicals such as polyolefins ( plastics), aromatics and synthetic resins. The origin of fossil fuels is the anaerobic decomposition of buried dead organisms. The conversion from these organic materials to high-carbon fossil fuels is ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
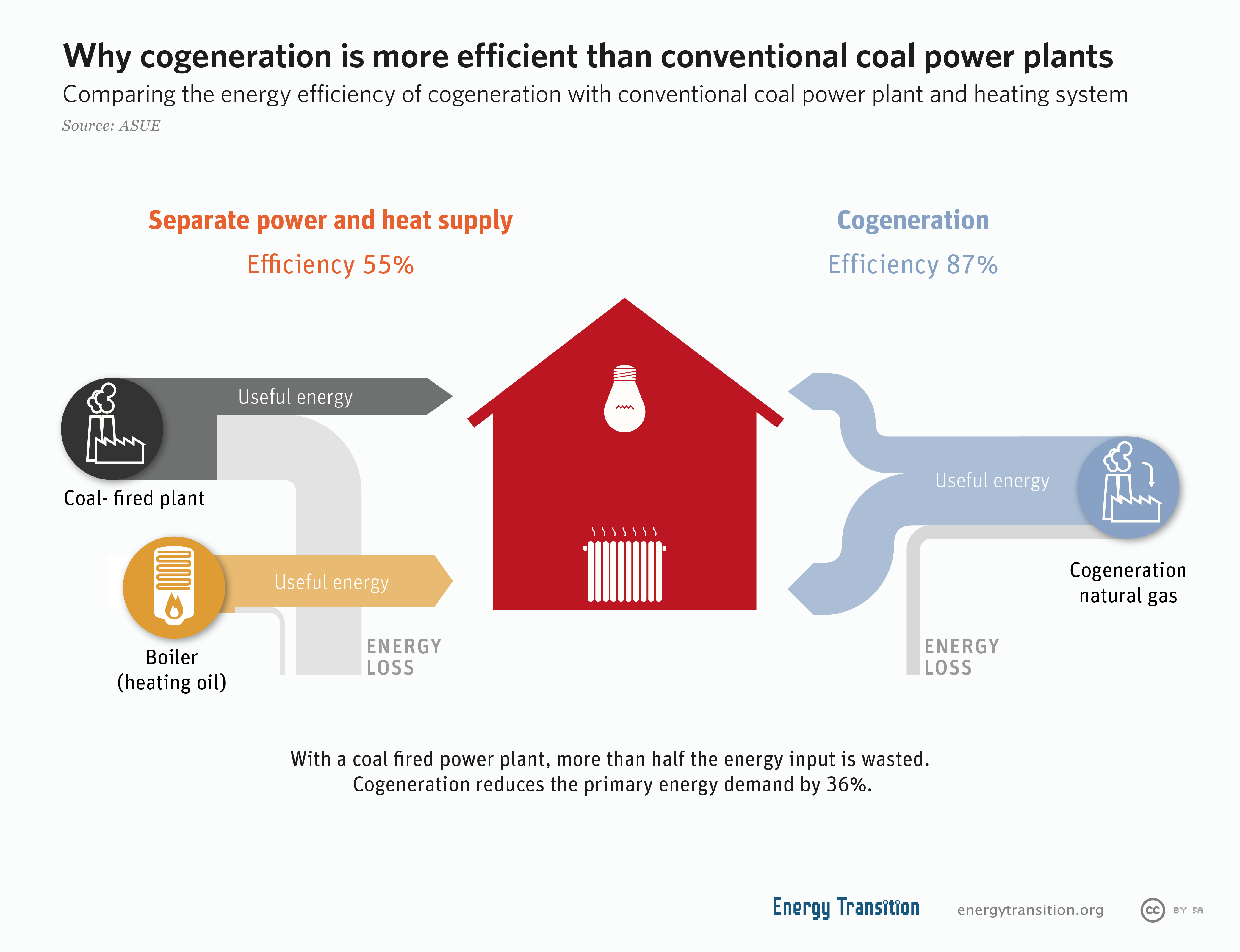
Cogeneration
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Cogeneration is a more efficient use of fuel or heat, because otherwise- wasted heat from electricity generation is put to some productive use. Combined heat and power (CHP) plants recover otherwise wasted thermal energy for heating. This is also called combined heat and power district heating. Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures ( can also be used in absorption refrigerators for cooling. The supply of high-temperature heat first drives a gas or steam turbine-powered generator. The resulting low-temperature waste heat is then used for water or space heating. At smaller scales (typically below 1 MW), a gas engine or diesel engine may be used. Cogeneration is also common with geothermal power plants as they often produce relatively low grade heat. Binary cycle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waste Heat
Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utility (or in thermodynamics lexicon a lower exergy or higher entropy) than the original energy source. Sources of waste heat include all manner of human activities, natural systems, and all organisms, for example, incandescent light bulbs get hot, a refrigerator warms the room air, a building gets hot during peak hours, an internal combustion engine generates high-temperature exhaust gases, and electronic components get warm when in operation. Instead of being "wasted" by release into the ambient environment, sometimes waste heat (or cold) can be used by another process (such as using hot engine coolant to heat a vehicle), or a portion of heat that would otherwise be wasted can be reused in the same process if make-up heat is added to the sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |