|
Molecular Chaos
In the kinetic theory of gases in physics, the molecular chaos hypothesis (also called ''Stosszahlansatz'' in the writings of Paul and Tatiana Ehrenfest) is the assumption that the velocities of colliding particles are uncorrelated, and independent of position. This means the probability that a pair of particles with given velocities will collide can be calculated by considering each particle separately and ignoring any correlation between the probability for finding one particle with velocity and probability for finding another velocity in a small region . James Clerk Maxwell introduced this approximation in 1867 although its origins can be traced back to his first work on the kinetic theory in 1860. The assumption of molecular chaos is the key ingredient that allows proceeding from the BBGKY hierarchy to Boltzmann's equation, by reducing the 2-particle distribution function showing up in the collision term to a product of 1-particle distributions. This in turn leads to Bol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Theory Of Gases
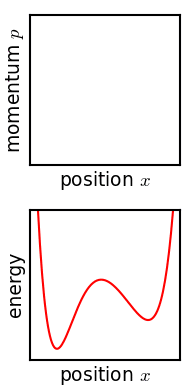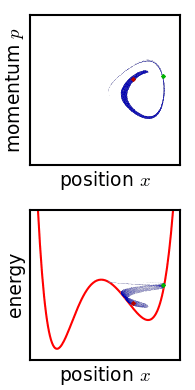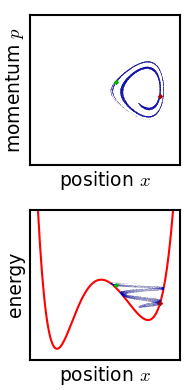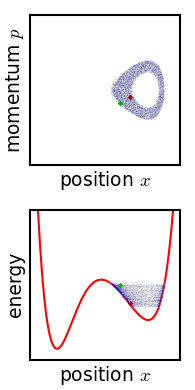
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small to be seen with a microscope, in constant, random motion. These particles are now known to be the atoms or molecules of the gas. The kinetic theory of gases uses their collisions with each other and with the walls of their container to explain the relationship between the macroscopic properties of gases, such as volume, pressure, and temperature, as well as transport properties such as viscosity, thermal conductivity and mass diffusivity. The basic version of the model describes an ideal gas. It treats the collisions as perfectly elastic and as the only interaction between the particles, which are additionally assumed to be much smaller than their average distance apart. Due to the time reversibility of microscopic dynamics ( microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Ehrenfest
Paul Ehrenfest (; 18 January 1880 – 25 September 1933) was an Austrian Theoretical physics, theoretical physicist who made major contributions to statistical mechanics and its relation to quantum physics, quantum mechanics, including the theory of phase transition and the Ehrenfest theorem. He befriended Albert Einstein on a visit to Prague in 1912 and became a professor in Leiden, where he frequently hosted Einstein. Suffering from depression, in 1933 Ehrenfest killed his disabled son, Wassik, and then himself. Biography Paul Ehrenfest was born on 18 January 1880 in Vienna to Ashkenazi Jews, Jewish parents, who were originally from Loštice in Moravia (now part of the Czech Republic). His parents, Sigmund Ehrenfest and Johanna Jellinek, managed a grocery store. Although the family was not overly religious, Paul studied Hebrew language, Hebrew and Jewish history. Later, he always emphasized his Jewish ancestry. Ehrenfest excelled in grade school but did not do well at the Akad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tatyana Afanasyeva
Tatyana Alexeyevna Afanasyeva-Ehrenfest (; ; 19 November 1876 – 14 April 1964) was a Russian-Dutch mathematician and physicist who made contributions to the fields of statistical mechanics and statistical thermodynamics with her husband Paul Ehrenfest. Early life Tatyana Afanasyeva was born in Kiev, Ukraine, then part of the Russian Empire. Her father was Alexander Afanassjev, a chief engineer on the Imperial Railways, who would bring Tatyana on his travels around the Russian Empire. Her father died while she was still young, so she moved to St Petersburg in Russia to live with her aunt Sonya, and uncle Peter Afanassjev, a professor at the St Petersburg Polytechnic Institute. Afanasyeva attended normal school in St Petersburg with a specialty in mathematics and science. At the time, women were not allowed to attend universities in Russian territory, so after graduating from normal school, Tatyana began studying mathematics and physics at the Women's University in St Pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism and light as different manifestations of the same phenomenon. Maxwell's equations for electromagnetism achieved the Unification (physics)#Unification of magnetism, electricity, light and related radiation, second great unification in physics, where Unification (physics)#Unification of gravity and astronomy, the first one had been realised by Isaac Newton. Maxwell was also key in the creation of statistical mechanics. With the publication of "A Dynamical Theory of the Electromagnetic Field" in 1865, Maxwell demonstrated that electric force, electric and magnetic fields travel through space as waves moving at the speed of light. He proposed that light is an undulation in the same medium that is the cause of electric and magnetic phenomena. (Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BBGKY Hierarchy
In statistical physics, the Bogoliubov–Born–Green–Kirkwood–Yvon (BBGKY) hierarchy (sometimes called Bogoliubov hierarchy) is a set of equations describing the dynamics of a system of a large number of interacting particles. The equation for an ''s''-particle distribution function (probability density function) in the BBGKY hierarchy includes the (''s'' + 1)-particle distribution function, thus forming a coupled chain of equations. This formal theoretic result is named after Nikolay Bogolyubov, Max Born, Herbert S. Green, John Gamble Kirkwood, and . Formulation The evolution of an ''N''-particle system in absence of quantum fluctuations is given by the Liouville equation for the probability density function f_N = f_N(\mathbf_1 \dots \mathbf_N, \mathbf_1 \dots \mathbf_N, t) in 6''N''-dimensional phase space (3 space and 3 momentum coordinates per particle) \frac + \sum_^N \frac \frac + \sum_^N \mathbf_i \frac = 0, where \mathbf_i, \mathbf_i are the position a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Equation
The Boltzmann equation or Boltzmann transport equation (BTE) describes the statistical behaviour of a thermodynamic system not in a state of Thermodynamic equilibrium, equilibrium; it was devised by Ludwig Boltzmann in 1872.Encyclopaedia of Physics (2nd Edition), Rita G. Lerner, R. G. Lerner, G. L. Trigg, VHC publishers, 1991, ISBN (Verlagsgesellschaft) 3-527-26954-1, ISBN (VHC Inc.) 0-89573-752-3. The classic example of such a system is a fluid with temperature gradients in space causing heat to flow from hotter regions to colder ones, by the random but biased transport of the particles making up that fluid. In the modern literature the term Boltzmann equation is often used in a more general sense, referring to any kinetic equation that describes the change of a macroscopic quantity in a thermodynamic system, such as energy, charge or particle number. The equation arises not by analyzing the individual position vector, positions and momentum, momenta of each particle in the fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H-theorem
In classical statistical mechanics, the ''H''-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency of the quantity ''H'' (defined below) to decrease in a nearly-ideal gas of molecules.L. Boltzmann,Weitere Studien über das Wärmegleichgewicht unter Gasmolekülen." Sitzungsberichte Akademie der Wissenschaften 66 (1872): 275-370. English translation: As this quantity ''H'' was meant to represent the entropy of thermodynamics, the ''H''-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics. It is thought to prove the second law of thermodynamics, albeit under the assumption of low-entropy initial conditions. The ''H''-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The ''H''-theorem has led to considerable discuss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loschmidt
Johann Josef Loschmidt (15 March 1821 – 8 July 1895), better known as Josef Loschmidt, was an Austrian scientist who performed ground-breaking work in chemistry, physics (thermodynamics, optics, electrodynamics), and crystal forms. Born in Karlsbad, a town in the Austrian Empire (now Karlovy Vary, Czech Republic), Loschmidt became professor of physical chemistry at the University of Vienna in 1868. He had two early mentors. The first was Bohemian priest Adalbert Czech, who persuaded Loschmidt's parents to send young Josef to high school in the Piarist monastery in Schlackenwerth and, in 1837, to advanced high-school classes in Prague. This was followed by two years of philosophy and mathematics at Prague's Charles University, where Loschmidt met his second important mentor. This was philosophy professor Franz Serafin Exner, whose eyesight was failing, and who asked Loschmidt to be his personal reader. Exner was known for his innovative school reforms, which included promot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irreversible Process
In thermodynamics, an irreversible process is a thermodynamic processes, process that cannot be undone. All complex natural processes are irreversible, although a phase transition at the coexistence temperature (e.g. melting of ice cubes in water) is well approximated as reversible. A change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. Because entropy is a state function, the change in entropy of the system is the same whether the process is reversible or irreversible. However, the impossibility occurs in restoring the Environment (systems), environment to its own initial conditions. An irreversible process increases the total entropy of the system and its surroundings. The second law of thermodynamics can be used to dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loschmidt's Paradox
In physics, Loschmidt's paradox (named for Josef Loschmidt), also known as the reversibility paradox, irreversibility paradox, or ' (), is the objection that it should not be possible to deduce an irreversible process from time-symmetric dynamics. This puts the time reversal symmetry of (almost) all known low-level fundamental physical processes at odds with any attempt to infer from them the second law of thermodynamics, which describes the behaviour of macroscopic systems. Both of these are well-accepted principles in physics, with sound observational and theoretical support, yet they seem to be in conflict, hence the paradox. Origin Josef Loschmidt's criticism was provoked by the H-theorem of Boltzmann, which employed kinetic theory to explain the increase of entropy in an ideal gas from a non-equilibrium state, when the molecules of the gas are allowed to collide. In 1876, Loschmidt pointed out that if there is a motion of a system from time ''t''0 to time ''t''1 to tim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principle Of Maximum Entropy
The principle of maximum entropy states that the probability distribution which best represents the current state of knowledge about a system is the one with largest entropy, in the context of precisely stated prior data (such as a proposition that expresses testable information). Another way of stating this: Take precisely stated prior data or testable information about a probability distribution function. Consider the set of all trial probability distributions that would encode the prior data. According to this principle, the distribution with maximal information entropy is the best choice. History The principle was first expounded by E. T. Jaynes in two papers in 1957, where he emphasized a natural correspondence between statistical mechanics and information theory. In particular, Jaynes argued that the Gibbsian method of statistical mechanics is sound by also arguing that the entropy of statistical mechanics and the information entropy of information theory are the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |