|
Miyaura Borylation
Miyaura borylation, also known as the Miyaura borylation reaction, is a named reaction in organic chemistry that allows for the generation of boronates from vinyl or aryl halides with the cross-coupling of bis(pinacolato)diboron in basic conditions with a catalyst such as PdCl2(dppf). The resulting borylated products can be used as coupling partners for the Suzuki reaction. Scope The Miyaura borylation has shown to work for: Alkyl halides, aryl halides, aryl halides using tetrahydroxydiboron, aryl halides using bis-boronic acid, aryl triflates, aryl mesylates, vinyl halides, vinyl halides of α,β-unsaturated carbonyl compounds, and vinyl triflates. See also * Chan-Lam coupling * Heck reaction * Hiyama coupling * Kumada coupling * Negishi coupling * Petasis reaction * Sonogashira coupling * Stille reaction * Suzuki reaction * List of organic reactions Well-known reactions and reagents in organic chemistry include 0-9 * 1,2-Wittig rearrangement * 1,3-Dipolar cycloadditio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norio Miyaura
was a Japanese organic chemist. He was a professor of graduate chemical engineering at Hokkaido University. His major accomplishments surrounded his work in cross-coupling reactions / conjugate addition reactions of organoboronic acids (for C-C bond-forming reactions) and addition / coupling reactions of diborons and boranes (to synthesize organoboronic acids and esters through B-C bond-forming reactions). He is also the co-author of ''Cross-Coupling Reactions: A Practical Guide'' with M. Nomura E. S.. Miyaura was a world-known and accomplished researcher by the time he retired and so, in 2007, he won the Japan Chemical Society Award. Early life and education Norio Miyaura was born in Hokkaido, Japan in 1946. Miyaura received his bachelors in chemical engineering from Hokkaido University in 1969. He next received his masters in chemical engineering from Hokkaido University in 1971. Lastly, he received his doctorate in chemical engineering from Hokkaido University in 1976. Care ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
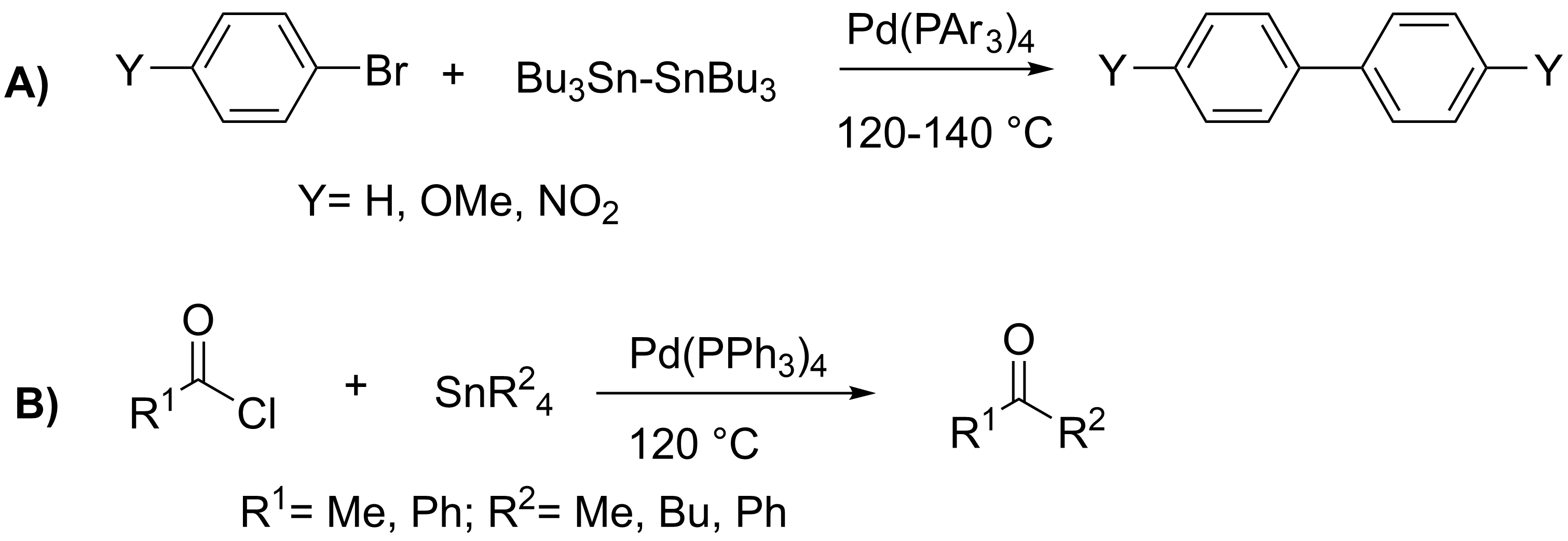
Stille Reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.Hartwig, J. F. ''Organotransition Metal Chemistry, from Bonding to Catalysis''; University Science Books: New York, 2010. Stille, J. K. '' Angew. Chem. Int. Ed. Engl.'' 1986, ''25'', 508–524.ReviewFarina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.Review : + \ \ce \ \overbrace^ + \!-\! :*\!,\ : Allyl, alkenyl, aryl, benzyl,acyl :*: halides (Cl, Br, I), pseudohalides (, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including vinyl, and aryl groups. These organostannanes are also stable to both air and moisture, and many of these reagents either are c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sonogashira Coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide. :* : aryl or vinyl :* : arbitrary :* X: I, Br, Cl or OTf The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon–carbon bonds. The reaction can be carried out under mild conditions, such as at room temperature, in aqueous media, and with a mild base, which has allowed for the use of the Sonogashira cross-coupling reaction in the synthesis of complex molecules. Its applications include pharmaceuticals, natural products, organic materials, and nanomaterials. Specific examples include its use in the synthesis of tazarotene, which is a treatment for psoriasis and acne, and in the preparation of SIB-1508Y, also known as Altinicline, a nicotinic recep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petasis Reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines. Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine. In the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery. Reaction scope and synthetic applications The amine is condensed with the carbonyl followed by addition of the boronic acid . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negishi Coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc. : :* The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions. :* The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl. :* The halide X' in the organozinc compound can be chloride, bromine or iodine and the organic residue R' is alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl. :* The metal M in the catalyst is nickel or palladium :* The ligand L in the catalyst can be triphenylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kumada Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki reaction, Suzuki, Sonogashira coupling, Sonogashira, Stille coupling, Stille, Hiyama coupling, Hiyama, Negishi coupling, Negishi), the Kumada coupling continues to be employed in many Chemical synthesis, synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hiyama Coupling
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds (C-C bonds). This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products. :\begin\\ \ce \end :* R: aryl, alkenyl or alkynyl :* R': aryl, alkenyl, alkynyl or alkyl :* R'': Cl, F or alkyl :* X: Cl, Br, I or OTf Reaction history The Hiyama coupling was developed to combat the issues associated with other organometallic reagents. The initial reactivity of organosilicon was not actually first reported by Hiyama, as Kumada reported a coupling reaction using organofluorosilicates shown below. Organosilanes were then discovered, by Hiyama, to have reactivity when activated by a fluoride source. This reactivity, when combined with a palladium s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Named Reaction
A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include the Grignard reaction, the Sabatier reaction, the Wittig reaction, the Claisen condensation, the Friedel-Crafts acylation, and the Diels-Alder reaction. Books have been published devoted exclusively to name reactions;Alfred Hassner, C. Stumer. ''Organic syntheses based on name reactions''. Elsevier, 2002. Li, Jie Jack. ''Name Reactions: A Collection of Detailed Reaction Mechanisms''. Springer, 2003. the Merck Index, a chemical encyclopedia, also includes an appendix on name reactions. As organic chemistry developed during the 20th century, chemists started associating synthetically useful reactions with the names of the discoverers or developers; in many cases, the name is merely a mnemonic. Some cases of reactions that were not reall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesylates
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group or anion, the spelling used is sometimes mesilate (as in ''imatinib mesilate'', the mesylate salt of imatinib). Mesylate esters are a group of organic compounds that share a common functional group with the general structure , abbreviated , where R is an organic substituent. Mesylate is considered a leaving group in nucleophilic substitution reactions. Preparation Mesylates are generally prepared by treating an alcohol and methanesulfonyl chloride in the presence of a base, such as triethylamine. Mesyl Related to mesylate is the mesyl (Ms) or methanesulfonyl (CH3SO2) functional group. Methanesulfonyl chloride is often referred to as mesyl chloride. Whereas mesylates are often hydrolytically labile, mesyl groups, when attached to nitro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |